Your How to do stoichiometry grams to moles images are ready. How to do stoichiometry grams to moles are a topic that is being searched for and liked by netizens now. You can Download the How to do stoichiometry grams to moles files here. Get all royalty-free vectors.
If you’re searching for how to do stoichiometry grams to moles images information connected with to the how to do stoichiometry grams to moles keyword, you have visit the ideal site. Our site always provides you with hints for downloading the highest quality video and picture content, please kindly surf and find more enlightening video content and graphics that match your interests.
How To Do Stoichiometry Grams To Moles. Find the GFM for the substance in this case H 2 O. For water the GFM is 1802 gmol. 35 moles H 2 O x 1802 grams 1 mole H 2 O 35 x 1802 grams 6307 grams. The unit is typically gmol.
 Molarity Dilution Problems Solution Stoichiometry Grams Moles Liters Volume Calculations Chemistry Y Stoichiometry Chemistry Chemistry Worksheets Chemistry From pinterest.com
Molarity Dilution Problems Solution Stoichiometry Grams Moles Liters Volume Calculations Chemistry Y Stoichiometry Chemistry Chemistry Worksheets Chemistry From pinterest.com
Find the molar mass from the formula. Using the molar mass as a conversion factor you can calculate the number of moles present in the stated. 35 moles H 2 O x 1802 grams1 mole H 2 O. Craig Beals explains the process of mole to mass stoichiometry in Chemistry. For this site we will use two decimal places when rounding the gram atomic mass from the periodic table. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula.
It contains mole to mole conversions grams to grams and mole to gram dimens.
Check to see if your answer makes sense. High School Chemistry Lesson. If 125 g Na are produced from the reaction how many moles of chlorine will be produced. 6 2 g r a m s H X 2 1 g r a m O X. Using the molar mass as a conversion factor you can calculate the number of moles present in the stated. N m M where M is the molar mass of this material.
 Source: pinterest.com
Source: pinterest.com
Find moles by diving given volume to molar volume. Using the molar mass as a conversion factor you can calculate the number of moles present in the stated. Determine the given and what is being asked. Follow the links below to do more practice. Find moles by diving given volume to molar volume.
 Source: pinterest.com
Source: pinterest.com
35 moles H 2 O x 1802 grams1 mole H 2 O. April 16 2020. Craig Beals explains the process of mole to mass stoichiometry in Chemistry. Set up the math in the following format. Mole conversion diagram this world is really awesome.
 Source: pinterest.com
Source: pinterest.com
For water the GFM is 1802 gmol. This chemistry video tutorial provides a basic introduction into stoichiometry. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. How do you use stoichiometry. So 9 grams should be a about one half of a mole.
 Source: pinterest.com
Source: pinterest.com
Or in plain English two moles of hydrogen and one mole of oxygen react to produce two moles of water. And these are steps to convert from liters of gas to grams of a gas. It contains mole to mole conversions grams to grams and mole to gram dimens. This is to ensure consistency in our calculations so we can compare numbers. Find the GFM for the substance in this case H 2 O.
 Source: pinterest.com
Source: pinterest.com
April 16 2020. Convert moles of wanted substance to desired units. And these are steps to convert from liters of gas to grams of a gas. When converting grams to moles. Convert moles of the wanted substance to the desired units.
 Source: pinterest.com
Source: pinterest.com
35 moles H 2 O x 1802 grams1 mole H 2 O. For example my periodic table reports hydrogen H as 10079 gmol. 962 of h 2 o. 962 grams H 2 O x 1 mole H 2 O1802 grams 962 x 1 mole 1802 053 moles. N m M where M is the molar mass of this material.
 Source: pinterest.com
Source: pinterest.com
Now lets use equation 1 and multiply each side by 1 gram s as we can do in algebra. Introduction to the mole graphic organizer teaching. Determine the given and what is being asked. This chemistry video tutorial provides a basic introduction into stoichiometry. Here we know that 1 mole of H 2 O is about 18 grams look at the GFM.
 Source: pinterest.com
Source: pinterest.com
Mole conversion diagram this world is really awesome. Set up the multiplication grid Put the given on the front ledge and what you want to find behind the grid. Mole conversion diagram this world is really awesome. Or in plain English two moles of hydrogen and one mole of oxygen react to produce two moles of water. Make sure your given is in moles If gramslitersparticles of A convert the moles of A.
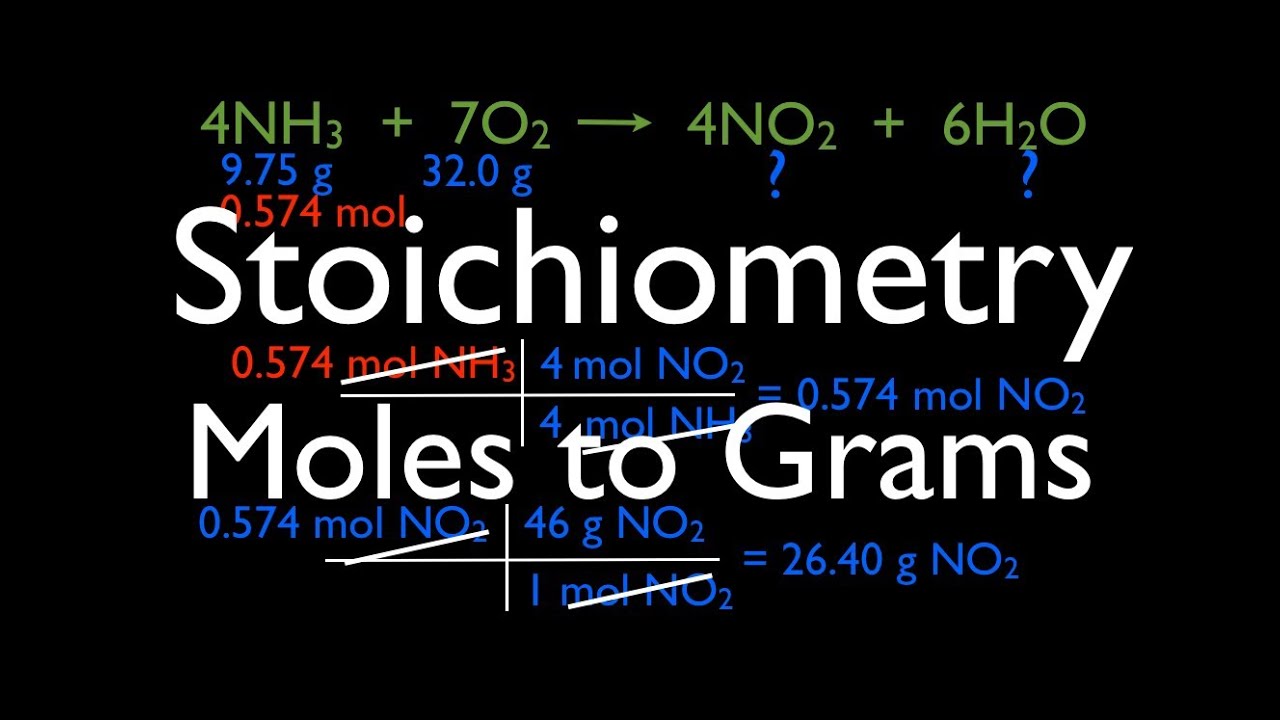 Source: pinterest.com
Source: pinterest.com
To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. Convert units of a given substance to moles. Convert moles of wanted substance to desired units. Gram to Gram Stoichiometry April 16 2020. The unit is typically gmol.
 Source: pinterest.com
Source: pinterest.com
Mole conversion diagram this world is really awesome. Using the mole 602 x 10 23 avogadros number one mole of molecules of watercontainsUsing the mole ratio calculate the moles of substance yielded by the reactionWe will use proportions to solve stoichiometry. 35 moles H 2 O x 1802 grams 1 mole H 2 O 35 x 1802 grams 6307 grams. Shows how to use stoichiometry to determine the number of moles of reactants and products if you are given the number of grams of one of the substances in th. Always have a balanced chemical equation.
 Source: pinterest.com
Source: pinterest.com
35 moles h 2 o x 1802 grams1 mole h 2 o. Convert moles of wanted substance to desired units. How do you convert liters to grams in stoichiometry. Tips for solving stoichiometry problems. Almost all stoichiometric problems can be solved in just four simple steps.
 Source: pinterest.com
Source: pinterest.com
Using the mole 602 x 10 23 avogadros number one mole of molecules of watercontainsUsing the mole ratio calculate the moles of substance yielded by the reactionWe will use proportions to solve stoichiometry. So 9 grams should be a about one half of a mole. The unit is typically gmol. Shows how to use stoichiometry to determine the number of moles of reactants and products if you are given the number of grams of one of the substances in th. Make sure your given is in moles If gramslitersparticles of A convert the moles of A.
 Source: pinterest.com
Source: pinterest.com
So 9 grams should be a about one half of a mole. Convert the units of the given substance a to molesConverting grams to moles step 1Divide the amount of the compound in grams by the molecular weight. For example my periodic table reports hydrogen H as 10079 gmol. It contains mole to mole conversions grams to grams and mole to gram dimens. Remember that the Avogadro constant is defined as the number of constituent particles atom or formula unit per mole of a given substance.
 Source: pinterest.com
Source: pinterest.com
Shows how to use stoichiometry to determine the number of moles of reactants and products if you are given the number of grams of one of the substances in th. If 125 g Na are produced from the reaction how many moles of chlorine will be produced. Find the smallest proportions of whole numbers among the warts of the elements. 02 H2 H20 and you have 010 mol of 02 just use the Avogadros number. For this site we will use two decimal places when rounding the gram atomic mass from the periodic table.
 Source: pinterest.com
Source: pinterest.com
For this site we will use two decimal places when rounding the gram atomic mass from the periodic table. Convert units of a given substance to moles. Always have a balanced chemical equation. Convert moles of wanted substance to desired units. Gram to Gram Stoichiometry April 16 2020.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to do stoichiometry grams to moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






