Your How to do moles to grams stoichiometry images are available in this site. How to do moles to grams stoichiometry are a topic that is being searched for and liked by netizens today. You can Download the How to do moles to grams stoichiometry files here. Download all free photos and vectors.
If you’re looking for how to do moles to grams stoichiometry pictures information connected with to the how to do moles to grams stoichiometry topic, you have come to the ideal blog. Our site frequently provides you with suggestions for viewing the maximum quality video and image content, please kindly hunt and find more informative video articles and images that match your interests.
How To Do Moles To Grams Stoichiometry. Id guess a bit less than 12 mole maybe 040 moles. In this case we have grams of BaCl 2. 1410 Gas Stoichiometry Practice Questions. Here we know that 1 mole of H.
 Stoichiometry Tutorial Step By Step Video Review Problems Explained Crash Chemistry Academy You Teaching Chemistry Chemistry Lessons Chemistry Worksheets From pinterest.com
Stoichiometry Tutorial Step By Step Video Review Problems Explained Crash Chemistry Academy You Teaching Chemistry Chemistry Lessons Chemistry Worksheets From pinterest.com
1410 Gas Stoichiometry Practice Questions. Gallium oxide decomposed to produce 012 grams of oxygen. How do you convert liters to grams in stoichiometry. Also remember that when we went from moles to grams we multiplied or mole-tiplied if it helps you remember. Converting from grams to moles well do the opposite. So we need to convert grams of BaCl 2 to moles of BaCl 2.
Find the molar mass from the formula.
And it is the ultimate factor that chemists use in talking about any substance. In this case we have grams of BaCl 2. Converting from grams to moles well do the opposite. So one mole of water has a mass of 16 11 18 grams. You want a formulation and a periodic desk. Using the molar mass as a conversion factor you can calculate the number of moles present in the stated number of grams of the species.
 Source: pinterest.com
Source: pinterest.com
Find mass by multiplying the number of moles to the molar mass. If 2437 grams of gallium formed what was the mass of gallium oxide that reacted. Gallium oxide decomposed to produce 012 grams of oxygen. You do this by multiplying the moles by the molar mass of the substance. How do you convert liters to grams in stoichiometry.
 Source: pinterest.com
Source: pinterest.com
So we need to convert grams of BaCl 2 to moles of BaCl 2. 35 moles H 2 O x 1802 grams1 mole H 2 O 35 x 1802 grams 6307 grams Check to see if you answer makes sense. Defining moles 1 mole of atoms has a mass equal to the atomic mass in grams 1 mole of formula units has a mass equal to the formula mass in grams. The amount that one mole of fuel occupies at STP 224Lmol Mole to Mole Ratio. How many grams of oxygen can be produced from 152 grams of gallium oxide.
 Source: pinterest.com
Source: pinterest.com
To calculate n the number of moles. And we determine this using the periodic table. Find the molar mass from the formula. How do you convert liters to grams in stoichiometry. 2KClO3— 2KCl 3O2.
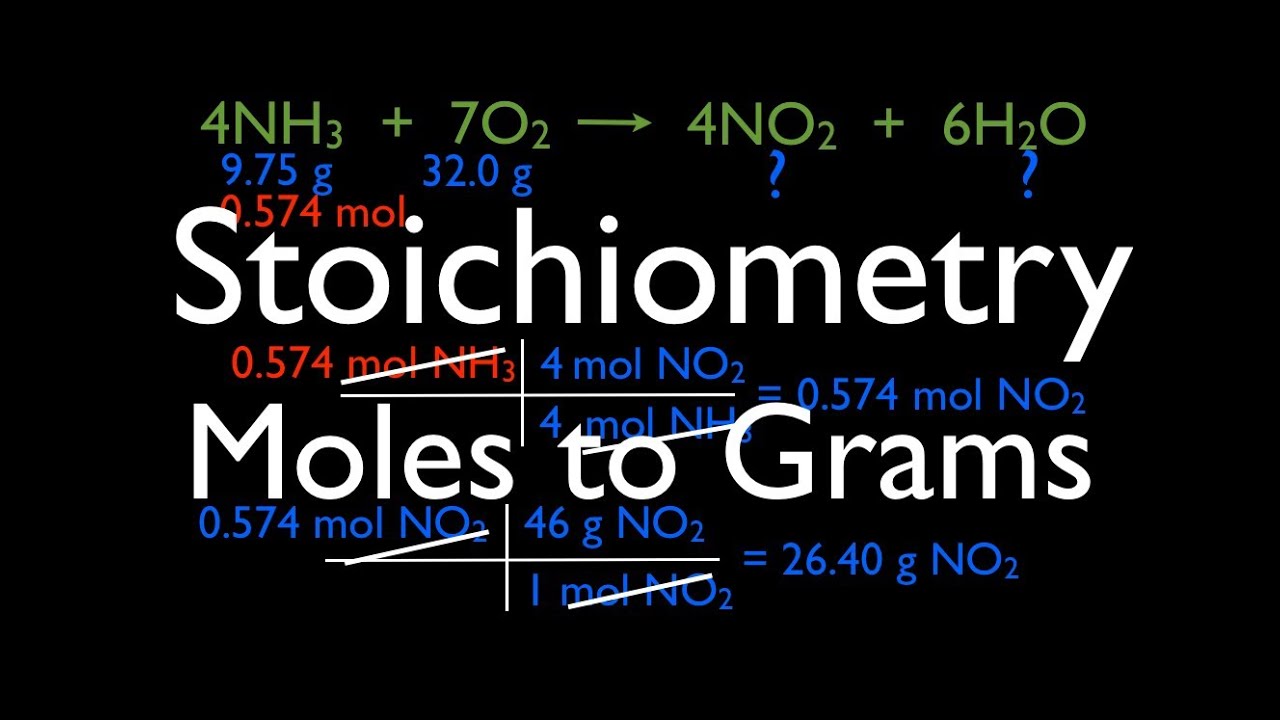 Source: pinterest.com
Source: pinterest.com
Is 500mg the same as 1 gram. How do you convert liters to grams in stoichiometry. Find moles by diving given volume to molar volume. What mass of gallium was produced. Find moles by diving given volume to molar volume.
 Source: pinterest.com
Source: pinterest.com
The molar mass for BaCl 2 is 208233 gmol. What mass of gallium was produced. The amount that one mole of fuel occupies at STP 224Lmol Mole to Mole Ratio. 35 moles h 2 o x 1802 grams1 mole h 2 o. Also remember that when we went from moles to grams we multiplied or mole-tiplied if it helps you remember.
 Source: gr.pinterest.com
Source: gr.pinterest.com
The right way to go from moles to grams in stoichiometry. In this case we have grams of BaCl 2. So if one mole has a mass of 18 grams 25 grams would have a mass of 25 grams 18 grams per mole or 139 moles. Find moles by diving given volume to molar volume. How to do stoichiometry calculations part1introduction.
 Source: pinterest.com
Source: pinterest.com
How to do stoichiometry calculations part1introduction. Find moles by diving given volume to molar volume. How do you convert liters to grams in stoichiometry. Is 500mg the same as 1 gram. Also remember that when we went from moles to grams we multiplied or mole-tiplied if it helps you remember.
 Source: pinterest.com
Source: pinterest.com
And it is the ultimate factor that chemists use in talking about any substance. Find mass by multiplying the number of moles to the molar mass. Id guess a bit less than 12 mole maybe 040 moles. This technique is covered in the mole section of the ChemTeam. And it is the ultimate factor that chemists use in talking about any substance.
 Source: pinterest.com
Source: pinterest.com
This technique is covered in the mole section of the ChemTeam. In case of oxygen the atomic mass of 16 gmol refers to 1 mol of o not o2 as molecular formIn performing mole to mole stoichiometry the first thing we need to do is to balance a chemical reactionJiggunjer is right that chemical equations tell you how much of each molecule you need in relation to others. And we determine this using the periodic table. Shows how to use stoichiometry to determine the number of grams of the reactants and products if you are given the number of moles of one substances in the c. Id guess a bit less than 12 mole maybe 040 moles.
 Source: pinterest.com
Source: pinterest.com
N m M the place. To do this we use molar mass. To calculate n the number of moles. You have the number of moles so you use the relationship gramsmoles to get the molar mass. You want a formulation and a periodic desk.
 Source: pinterest.com
Source: pinterest.com
Also remember that when we went from moles to grams we multiplied or mole-tiplied if it helps you remember. 35 moles h 2 o x 1802 grams1 mole h 2 o. The amount that one mole of fuel occupies at STP 224Lmol Mole to Mole Ratio. To calculate n the number of moles. Grams to mg conversion 1 gram g is equal to 1000 milligrams mg.
 Source: pinterest.com
Source: pinterest.com
In performing mole to mole stoichiometry the first thing we need to do is to balance a chemical reaction. This technique is covered in the mole section of the ChemTeam. And these are steps to convert from liters of gas to grams of a gas. Grams to mg conversion 1 gram g is equal to 1000 milligrams mg. Here we know that 1 mole of H.
 Source: pinterest.com
Source: pinterest.com
The amount that one mole of fuel occupies at STP 224Lmol Mole to Mole Ratio. You do this by multiplying the moles by the molar mass of the substance. To do this we use molar mass. Here is the equation well use for the first three examples. The gas is ideal.
 Source: pinterest.com
Source: pinterest.com
So one mole of water has a mass of 16 11 18 grams. Id guess a bit less than 12 mole maybe 040 moles. Using the molar mass as a conversion factor you can calculate the number of moles present in the stated number of grams of the species. And these are steps to convert from liters of gas to grams of a gas. Make sure your given is in moles If gramslitersparticles of A convert the moles of A.
 Source: pinterest.com
Source: pinterest.com
Find the molar mass from the formula. To do this we use molar mass. Shows how to use stoichiometry to determine the number of grams of the reactants and products if you are given the number of moles of one substances in the c. How to do stoichiometry calculations part1introduction. Id guess a bit less than 12 mole maybe 040 moles.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to do moles to grams stoichiometry by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






