Your How to do grams to moles stoichiometry images are available. How to do grams to moles stoichiometry are a topic that is being searched for and liked by netizens today. You can Find and Download the How to do grams to moles stoichiometry files here. Find and Download all free photos and vectors.
If you’re looking for how to do grams to moles stoichiometry pictures information connected with to the how to do grams to moles stoichiometry interest, you have come to the right blog. Our site always provides you with hints for downloading the maximum quality video and image content, please kindly search and find more enlightening video content and images that fit your interests.
How To Do Grams To Moles Stoichiometry. Convert the units of the given substance a to molesConverting grams to moles step 1Divide the amount of the compound in grams by the molecular weight. How to Convert Grams to Moles. And these are steps to convert from liters of gas to grams of a gas. Make sure you are working with a properly balanced chemical equation.
 A Mole Conversion Is When The Mole Is Able The Measure Both Mass And Particles Chemistry Basics Organic Chemistry Study Chemistry From pinterest.com
A Mole Conversion Is When The Mole Is Able The Measure Both Mass And Particles Chemistry Basics Organic Chemistry Study Chemistry From pinterest.com
N m M where M is the molar mass of this material. Now lets use equation 1 and multiply each side by 1 gram s as we can do in algebra. Watch video circle and label what you are given and what you are trying to find. How To Do Stoichiometry Grams To Moles. Since moles grams of compoundthe molar mass to find the mass you will need to do mass molar mass x molarity. 02 H2 H20 and you have 010 mol of 02 just use the Avogadros number.
02 2 H2 2 H20 1 mol of oxygen is needed to react with 2 moles of Hydrogen to form 2 moles of Water.
The ratios can be based on mass moles volume. 962 grams H 2 O x 1 mole H 2 O1802 grams 962 x 1 mole 1802 053 moles. Find the smallest proportions of whole numbers among the warts of the elements. Shows how to use stoichiometry to determine the number of moles of reactants and products if you are given the number of grams of one of the substances in th. There are four steps involved in solving these problems. Make sure you are working with a properly balanced chemical equation.
 Source: pinterest.com
Source: pinterest.com
Gram to Gram Stoichiometry April 16 2020. Convert the units of the given substance A to moles. Gram to Gram Stoichiometry April 16 2020. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. Subsequently question is what is 1 mole in grams.
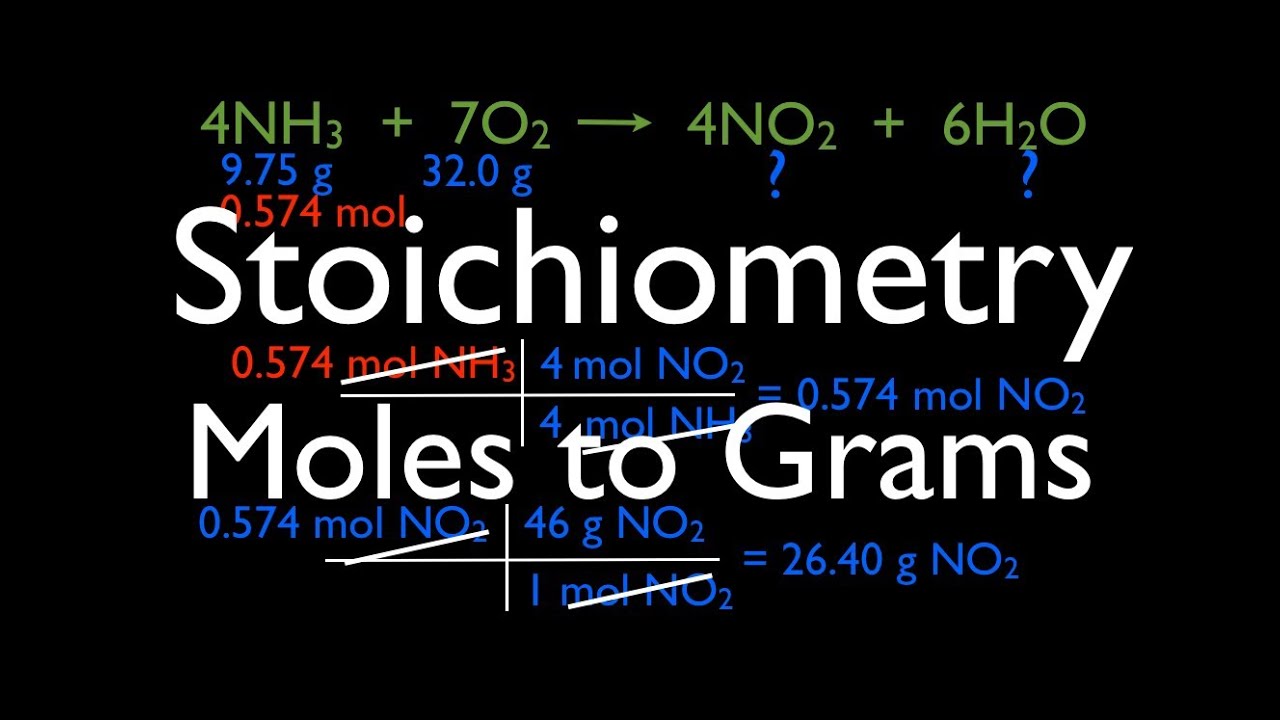 Source: pinterest.com
Source: pinterest.com
So 9 grams should be a about one half of a mole. Remember that the Avogadro constant is defined as the number of constituent particles atom or formula unit per mole of a given substance. If 125 g Na are produced from the reaction how many moles of chlorine will be produced. In this case we have grams of BaCl 2. Subsequently question is what is 1 mole in grams.
 Source: pinterest.com
Source: pinterest.com
Construct two ratios - one from the problem and. High School Chemistry Lesson. Make sure you are working with a properly balanced chemical equation. If 125 g Na are produced from the reaction how many moles of chlorine will be produced. And we determine this using the periodic table.
 Source: pinterest.com
Source: pinterest.com
Or in plain English two moles of hydrogen and one mole of oxygen react to produce two moles of water. So 9 grams should be a about one half of a mole. Or in plain English two moles of hydrogen and one mole of oxygen react to produce two moles of water. How many grams of sodium chloride are required to make 032 moles of chlorine. Stoichiometry Calculator- Formulas Problems Applications 2 days ago Triple Integral calculator- equation meaning formula 3 days ago.
 Source: pinterest.com
Source: pinterest.com
How many grams of sodium chloride are required to make 032 moles of chlorine. 962 grams H 2 O x 1 mole H 2 O1802 grams 962 x 1 mole 1802 053 moles. To do this we use molar mass. Watch video circle and label what you are given and what you are trying to find. How many grams of sodium chloride are required to make 032 moles of chlorine.
 Source: pinterest.com
Source: pinterest.com
And we determine this using the periodic table. Convert the units of the given substance A to moles. Or in plain English two moles of hydrogen and one mole of oxygen react to produce two moles of water. And these are steps to convert from liters of gas to grams of a gas. Make sure you are working with a properly balanced chemical equation.
 Source: pinterest.com
Source: pinterest.com
How To Do Stoichiometry Grams To Moles. N m M where M is the molar mass of this material. So 9 grams should be a about one half of a mole. To do this we use molar mass. And we determine this using the periodic table.
 Source: pinterest.com
Source: pinterest.com
If 125 g Na are produced from the reaction how many moles of chlorine will be produced. When converting grams to moles. Make sure your given is in moles If gramslitersparticles of A convert the moles of A. Watch video circle and label what you are given and what you are trying to find. Gram to Gram Stoichiometry April 16 2020.
 Source: pinterest.com
Source: pinterest.com
How many grams of sodium chloride are required to make 032 moles of chlorine. Follow the links below to do more practice. April 16 2020. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. Stoichiometry is an important principle in chemistry as it deals with ratios of amounts of chemicals in chemical reactions.
 Source: pinterest.com
Source: pinterest.com
Watch video circle and label what you are given and what you are trying to find. Find the smallest proportions of whole numbers among the warts of the elements. Construct two ratios - one from the problem and. High School Chemistry Lesson. Shows how to use stoichiometry to determine the number of moles of reactants and products if you are given the number of grams of one of the substances in th.
 Source: pinterest.com
Source: pinterest.com
Watch video circle and label what you are given and what you are trying to find. When converting grams to moles. Now lets use equation 1 and multiply each side by 1 gram s as we can do in algebra. How do you convert liters to grams in stoichiometry. Follow the links below to do more practice.
 Source: pinterest.com
Source: pinterest.com
See explanation to convert grams to moles you will need to molar mass of that specific element molar mass is found my multiplying the number of atoms present in the compound by the relative atomic mass found on the bottom of the. When converting grams to moles. Convert moles of the wanted substance to the desired units. In this case we have grams of BaCl 2. Make sure you are working with a properly balanced chemical equation.
 Source: pinterest.com
Source: pinterest.com
Using the molar mass as a conversion factor you can calculate the number of moles present in the stated number of. Find moles by diving given volume to molar volume. After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive. And these are steps to convert from liters of gas to grams of a gas. April 16 2020.
 Source: pinterest.com
Source: pinterest.com
The ratios can be based on mass moles volume. Remember that the Avogadro constant is defined as the number of constituent particles atom or formula unit per mole of a given substance. 02 2 H2 2 H20 1 mol of oxygen is needed to react with 2 moles of Hydrogen to form 2 moles of Water. See explanation to convert grams to moles you will need to molar mass of that specific element molar mass is found my multiplying the number of atoms present in the compound by the relative atomic mass found on the bottom of the. Write the balanced chemical equation.
 Source: pinterest.com
Source: pinterest.com
Subsequently question is what is 1 mole in grams. There are four steps in solving a stoichiometry problem. See explanation to convert grams to moles you will need to molar mass of that specific element molar mass is found my multiplying the number of atoms present in the compound by the relative atomic mass found on the bottom of the. April 16 2020. Convert grams of the substance given in the problem to moles.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to do grams to moles stoichiometry by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






