Your How to determine the number of moles in an element images are ready in this website. How to determine the number of moles in an element are a topic that is being searched for and liked by netizens today. You can Get the How to determine the number of moles in an element files here. Find and Download all free photos and vectors.
If you’re looking for how to determine the number of moles in an element pictures information connected with to the how to determine the number of moles in an element topic, you have visit the ideal blog. Our site always provides you with suggestions for viewing the highest quality video and image content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
How To Determine The Number Of Moles In An Element. Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance. So convert your moles of molecules to moles of atoms by multiplying by the eight coubt them atoms per molecule. 100mol of carbon-12 atoms has a mass of 120g and contains 60221023 atoms. How many atoms are in a glass of water.
 7 1 The Mole Concept Introductory Chemistry From courses.lumenlearning.com
7 1 The Mole Concept Introductory Chemistry From courses.lumenlearning.com
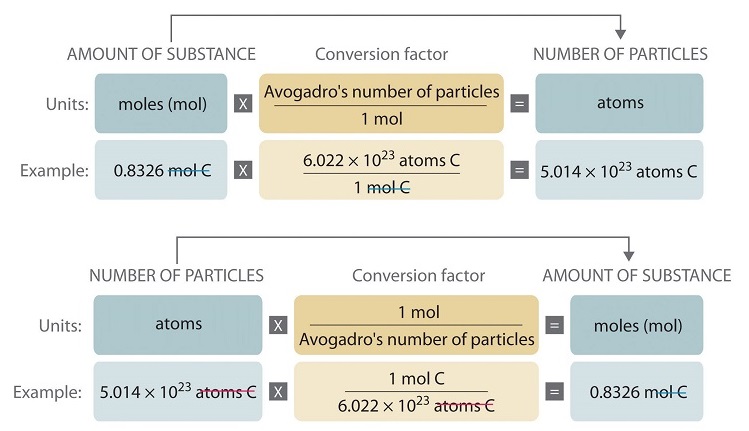
X moles 6022 10 23 atoms 1 mole y atoms For example if scientists want to know how may atoms are in six moles of sodium x 6 they could solve. Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance. So for every one mole of glucose C6H12O6 we have 18016 grams of glucose C6H12O6 and this is going to get us we get 152 times 1000 is equal to this is the number of grams of glucose we have and then were going to divide by 18016 divide by 18016 gives us this number and lets see if we see significant figures we have three. So convert your moles of molecules to moles of atoms by multiplying by the eight coubt them atoms per molecule. Molar mass is the number of grams in one mole 602 x 10. Water consists of one oxygen atom and two hydrogen atoms.
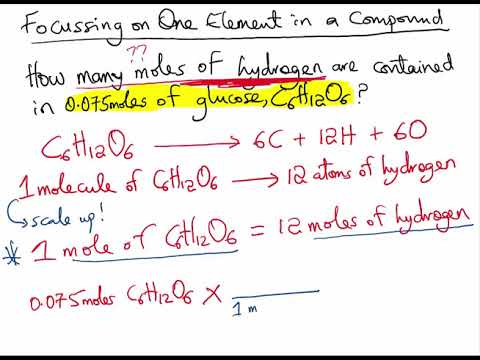
Here it takes 1 mole of K and 1 mole of Cl to make 1 mole of KCl because KCl is a bigger molecule than K or Cl and one molecule needs exactly 1 K and 1 Cl atom.
9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. Given a known number of moles x one can find the number of atoms y in this molar quantity by multiplying it by Avogadros number. X moles 6022 10 23 atoms 1 mole y atoms For example if scientists want to know how may atoms are in six moles of sodium x 6 they could solve. Number of mol is calculated by ratio of given mass to the molar mass. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. So convert your moles of molecules to moles of atoms by multiplying by the eight coubt them atoms per molecule.
 Source: youtube.com
Source: youtube.com
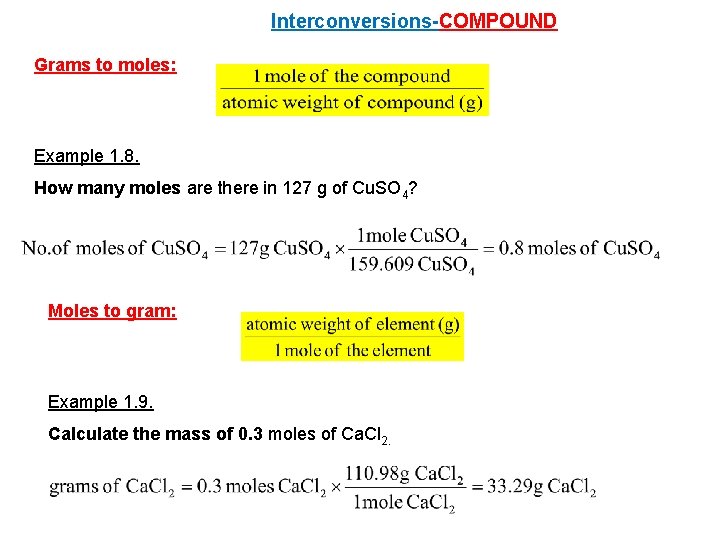
So for every one mole of glucose C6H12O6 we have 18016 grams of glucose C6H12O6 and this is going to get us we get 152 times 1000 is equal to this is the number of grams of glucose we have and then were going to divide by 18016 divide by 18016 gives us this number and lets see if we see significant figures we have three. This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles. The answer is the number of moles of that mass of compound. Lets say the question is How many oxygen atoms are in 123 g of KClO3. 100mol of carbon-12 atoms has a mass of 120g and contains 60221023 atoms.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Its all based on the definition. M r 1K 1Cl 3O 13910 13545 31600 12255. Get the chemical formula so that you can determine how many atoms of each element are present in the molecule. Given a known number of moles x one can find the number of atoms y in this molar quantity by multiplying it by Avogadros number. Water consists of one oxygen atom and two hydrogen atoms.
 Source: chem.libretexts.org
Source: chem.libretexts.org
In this manner how do you find the number of moles of an element in a compound. M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1. Molar mass is the number of grams in one mole 602 x 10. 6 moles 6022 1023 atoms 1 mole 361 10 24 atoms Note that the. Number of mol of sodium dfractextgiven masstextmolar mass.
 Source: slidetodoc.com
Source: slidetodoc.com
N 1 mole of N for 1 mole of KNO3. Follow this answer to receive notifications. If m is the mass of the compound in grams and M is the AMU mass of the compound there are N A m M molecules of the compound where N A is Avogadros number. Molar mass is the number of grams in one mole 602 x 10. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it.
 Source: khanacademy.org
Source: khanacademy.org
It can be used to measure the products obtained from the chemical reaction. 6 moles 6022 1023 atoms 1 mole 361 10 24 atoms Note that the. If you have 1000 grams. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. It can be used to measure the products obtained from the chemical reaction.
 Source: slideshare.net
Source: slideshare.net
To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. How many atoms are in a glass of water. Its all based on the definition. This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles. Water consists of one oxygen atom and two hydrogen atoms.
 Source: youtube.com
Source: youtube.com
This is thoroughly answered here. You calculate the number of moles by dividing the mass of substance by the substances atomic or molecular weight. So 1 mol of KClO3 has a mass of 12255 g. Water consists of one oxygen atom and two hydrogen atoms. Now to calculate its molar mass we add up all of the molar masses of each atom.
 Source: study.com
Source: study.com
This means that the mass of a water molecule is 1g 1g 16g. Follow this answer to receive notifications. Apr 30 2018 Divide the mass of the compound in grams by the molar mass you just calculated. Answered Mar 4 14 at 2348. To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3.
 Source: toppr.com
Source: toppr.com
First weigh the compound. To count individual atoms you have to multiply the percentage composition which I will say more about below by the total number of moles of atoms. How do you find the number of moles in a compound. So for finding out the molar mass of the molecule you have to break it into elements to know its molar mass. 100mol of carbon-12 atoms has a mass of 120g and contains 60221023 atoms.
 Source: youtube.com
Source: youtube.com
Answered Mar 4 14 at 2348. This means that the mass of a water molecule is 1g 1g 16g. Then 1000 g 151001 gmol X g moles. It doesnt matter how big or small the molecule is just how many of them there are. Number of mol is calculated by ratio of given mass to the molar mass.
 Source: youtube.com
Source: youtube.com
Molar mass is the number of grams in one mole 602 x 10. 1 mol of atoms 6022 1023latoms. How to calculate number of moles of specific element in a co. So convert your moles of molecules to moles of atoms by multiplying by the eight coubt them atoms per molecule. Calculate he number of moles you have by taking the Mass molar mass.
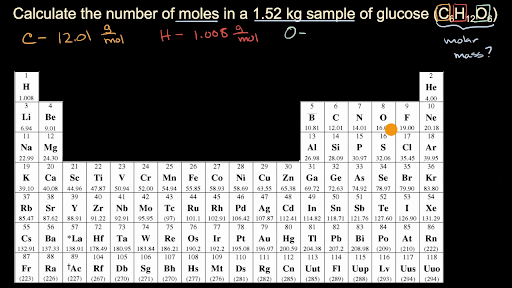
It doesnt matter how big or small the molecule is just how many of them there are. After calculating the number of moles the number of ions will be equal to the product of the number of moles and Avogadros number. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. Also the periodic table can help you to find the molar mass of each element. How to calculate number of moles of specific element in a co.
 Source: studylib.net
Source: studylib.net
100mol of carbon-12 atoms has a mass of 120g and contains 60221023 atoms. First when you get the number of moles 27971 you have the number of moles of molecules. If m is the mass of the compound in grams and M is the AMU mass of the compound there are N A m M molecules of the compound where N A is Avogadros number. Its all based on the definition. Now to calculate its molar mass we add up all of the molar masses of each atom.
 Source: studylib.net
Source: studylib.net
The unit is denoted by mol. How do you find the number of moles in a compound. So convert your moles of molecules to moles of atoms by multiplying by the eight coubt them atoms per molecule. One mole of any substance is equal to the value of 6023 x 1023 Avagadro number. The answer is the number of moles of that mass of compound.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Number of moles 112 Number of molecules 6745 10 24 and Number of atoms 1349 10 25 Science Chemistry Molecule and Molecular Mass Calculation of Number of Moles Atoms and Molecules. Water consists of one oxygen atom and two hydrogen atoms. This means that the mass of a water molecule is 1g 1g 16g. To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. So 1 mol of KClO3 has a mass of 12255 g.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to determine the number of moles in an element by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






