Your How to determine the moles of an element images are ready. How to determine the moles of an element are a topic that is being searched for and liked by netizens now. You can Download the How to determine the moles of an element files here. Get all free photos and vectors.
If you’re looking for how to determine the moles of an element images information related to the how to determine the moles of an element keyword, you have pay a visit to the right site. Our site always gives you suggestions for viewing the highest quality video and picture content, please kindly hunt and locate more enlightening video content and graphics that match your interests.
How To Determine The Moles Of An Element. Using the conversion factor we can calculate the moles of each element. K - 00495 mol. Convert the mass of each element to moles using the molar mass from the periodic table. So thats equal to 18016 grams per mole.
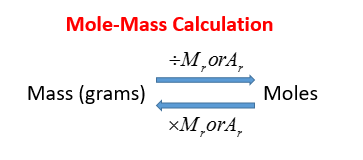 Mole Calculation Video Lessons Examples And Solutions From onlinemathlearning.com
Mole Calculation Video Lessons Examples And Solutions From onlinemathlearning.com
If you had 350 L of CO at STP then you would have 350 L 1 mol224 L 156 mol of CO Lastly it has been calculated that one mole of any substance contains 602 1023 atoms if you are talking about pure elements molecules if you are talking about covalent compounds or the general term particles used primarily for inorganic compounds. Calculate the number of grams per mole gmol for each reactant and product. How do you find the moles of an element in a compound. I can only go to the hundredths place for significant figures so 18016. N - 00495 mol. Calculate the moles of each element in C 12 H 22 O 11 and record your results in Table 2.
How to calculate number of moles of specific element in a co.
To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. For example the Chlorine Cl has a molar mass of 354530 gmol in the same way Sodium Na has a molar mass of 229898 gmol. Moles of element n moles elementone mole of compound K - 1 mole of K for 1 mole of KNO3. Jeremiah Daniels on How To Find Moles Of An Element In A Compound Given Mass. Example atoms in 12 grams are the same as 12C. Start with the number of grams of each element given in the problem.
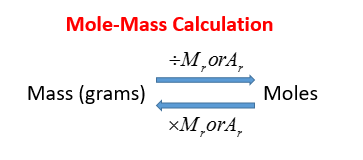 Source: onlinemathlearning.com
Source: onlinemathlearning.com
For example if scientists want to know how may atoms are in six moles of sodium x 6 they could solve. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. So thats equal to 18016 grams per mole. Moreover most of the molecules are made up of. 1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 1022 ions and the number of moles of NH4 ions 00301 moles.
 Source: khanacademy.org
Source: khanacademy.org
A mole fraction indicates the number of chemical elements. Find the Molar Mass of Adrenaline. Jeremiah Daniels on How To Find Moles Of An Element In A Compound Given Mass. Moles of element n moles elementone mole of compound K - 1 mole of K for 1 mole of KNO3. If you had 350 L of CO at STP then you would have 350 L 1 mol224 L 156 mol of CO Lastly it has been calculated that one mole of any substance contains 602 1023 atoms if you are talking about pure elements molecules if you are talking about covalent compounds or the general term particles used primarily for inorganic compounds.
 Source: slideplayer.com
Source: slideplayer.com
Molar Mass of Compound. The keys to getting the correct answer are. A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons. Make sure the chemical equation is balanced. The molar mass for carbon is 120 g.
 Source: dummies.com
Source: dummies.com
Note that the solution is independent of whether the element is sodium or otherwise. A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons. Add up the masses of the elements in each compound to find the grams per mole for that compound. The keys to getting the correct answer are. How to calculate number of moles of specific element in a co.
 Source: surfguppy.com
Source: surfguppy.com
Jeremiah Daniels on How To Find Moles Of An Element In A Compound Given Mass. This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles. Example atoms in 12 grams are the same as 12C. How do you find the moles of an element in a compound. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms.
 Source: youtube.com
Source: youtube.com
Using the conversion factor we can calculate the moles of each element. So thats equal to 18016 grams per mole. How do you find the moles of an element in a compound. Calculate the moles of C 12 H 22 O 11 contained in the sample and record your results in Table 2. First of all every element has a different molar mass and is expressed as gram per mole.
 Source: slideplayer.com
Source: slideplayer.com
N - 00495 mol. Calculate the number of atoms in 245 mol of copper. In other words 1 moles of carbon has a mass of 120 g. Jeremiah Daniels on How To Find Moles Of An Element In A Compound Given Mass. Molar Mass of Compound.
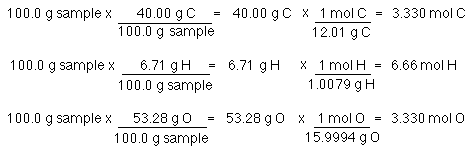 Source: westfield.ma.edu
Source: westfield.ma.edu
How do you find the moles of an element in a compound. 1 One mole of particles of any substance contains how many particles. Note that the solution is independent of whether the element is sodium or otherwise. To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. Example atoms in 12 grams are the same as 12C.
 Source: youtube.com
Source: youtube.com
Make sure the chemical equation is balanced. Moles Sn 788 g Sn x 1 mol Sn 1187 g Sn 0664 mol Sn moles O 212 g O x 1 mol O 1600 g O 133 mol O. M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l. A Mole Of Any Substance Contains The Same Number Of Atoms. Calculate the moles of each element in C 12 H 22 O 11 and record your results in Table 2.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
Start with the number of grams of each element given in the problem. Molar Mass of Compound. 6 moles 60221023 atoms 1 mole 3611024 atoms 6 moles 6022 10 23 atoms 1 mole 361 10 24 atoms. What Is A Mole. Formula to calculate moles.
 Source: xaktly.com
Source: xaktly.com
How To Calculate Moles In Chemistry. Moles Sn 788 g Sn x 1 mol Sn 1187 g Sn 0664 mol Sn moles O 212 g O x 1 mol O 1600 g O 133 mol O. If you had 350 L of CO at STP then you would have 350 L 1 mol224 L 156 mol of CO Lastly it has been calculated that one mole of any substance contains 602 1023 atoms if you are talking about pure elements molecules if you are talking about covalent compounds or the general term particles used primarily for inorganic compounds. Now to calculate its molar mass we add up all of the molar masses of each atom. The mole is important because it allows chemists to work with the subatomic world with macro world units and amounts.
 Source: youtube.com
Source: youtube.com
Check to make sure you use the appropriate number of significant figures for atomic masses and report mass using the correct number of figures. One mole of any substance is equal to the value of 6023 x 1023 Avagadro number. Now to calculate its molar mass we add up all of the molar masses of each atom. Moles of element n moles elementone mole of compound K - 1 mole of K for 1 mole of KNO3. The basic units can be molecules atoms or formula units based on the substance.
 Source: youtube.com
Source: youtube.com
Divide each mole value by the smallest number of. How do you find the moles of an element in a compound. O - 300495 mol 0148 mol. I can only go to the hundredths place for significant figures so 18016. Molar massis the atomic mass of an element expressed in grams Ex.
 Source: chem.fsu.edu
Source: chem.fsu.edu
Jeremiah Daniels on How To Find Moles Of An Element In A Compound Given Mass. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. Divide each mole value by the smallest number of. Molar Mass of Compound. How do you find the moles of an element in a compound.
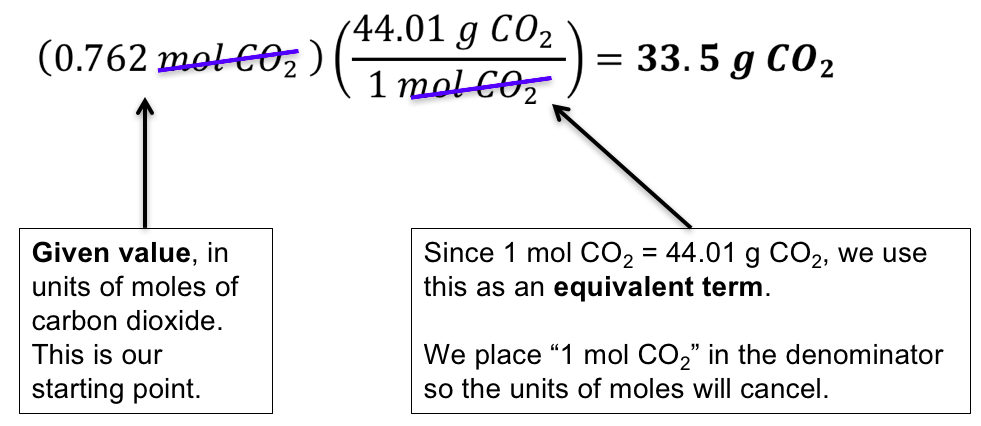 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. The molar mass for carbon is 120 g. One mole of any substance is equal to the value of 6023 x 1023 Avagadro number. Add up the masses of the elements in each compound to find the grams per mole for that compound. Divide each mole value by the smallest number of.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to determine the moles of an element by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






