Your How to determine moles of a compound images are available in this site. How to determine moles of a compound are a topic that is being searched for and liked by netizens today. You can Find and Download the How to determine moles of a compound files here. Find and Download all royalty-free photos and vectors.
If you’re searching for how to determine moles of a compound images information related to the how to determine moles of a compound keyword, you have visit the right blog. Our site always gives you suggestions for seeing the highest quality video and image content, please kindly hunt and locate more informative video content and images that match your interests.
How To Determine Moles Of A Compound. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. 16 g C 6 H 4 CO 2 H CO 2 CH 3 moles aspirin 180 x 10-3 mol aspirin. From compound to compound to a number of atoms in a molecule vary. The answer is the number of.
 Empirical And Molecular Formula Notes Chemistry Worksheets Chemistry Notes Teaching Chemistry From pinterest.com
Empirical And Molecular Formula Notes Chemistry Worksheets Chemistry Notes Teaching Chemistry From pinterest.com
Number of moles Weight of compound in grams molecular weight of compound. The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol. Calculating Molar Mass And Quantity Of Moles Labored Instance Video Khan Academy. The answer is the number of moles of that mass of compound. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4.
Calculating Molar Mass And Quantity Of Moles Labored Instance Video Khan Academy.
Number of moles Mass of substance Mass of one mole. Calculate the moles of each element in C 12 H 22 O 11 and record your results in Table 2. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. Divide the mass of the compound in grams by the molar mass you just calculated. Calculating The Moles Of An Aspect In A Compound Youtube. Calculate the moles of aspirin.
 Source: pinterest.com
Source: pinterest.com
Number of moles 95 8694. Determine the number of moles in 95g of MnO2. One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. Divide the mass of the compound in grams by the molar mass you just calculated. Calculating Molar Mass And Quantity Of Moles Labored Instance Video Khan Academy.
 Source: pinterest.com
Source: pinterest.com
Calculate the moles of C 12 H 22 O 11 contained in the sample and record your results in Table 2. There are three hydrogen atoms as indicated by the subscript. To go from grams to moles divide the grams by the molar mass. It contains plenty of examples and practice problemsMy E-Book. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl.
 Source: pinterest.com
Source: pinterest.com
Experts are tested by Chegg as specialists in their subject area. Number of moles formula is. Mass of MnO2 95g. Numerically this would be 2 1008 1 1600 18016. How do you find the moles of an element in a compound.
 Source: pinterest.com
Source: pinterest.com
One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. Number of moles Mass of substance Mass of one mole. One mole consists of Avogadro number of atoms. Mass of MnO2 95g. Number of moles formula is.
 Source: pinterest.com
Source: pinterest.com
There is one nitrogen atom no subscript is given for one atom. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. 16 g C 6 H 4 CO 2 H CO 2 CH 3 moles aspirin 180 x 10-3 mol aspirin. Number of moles formula is. The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol.
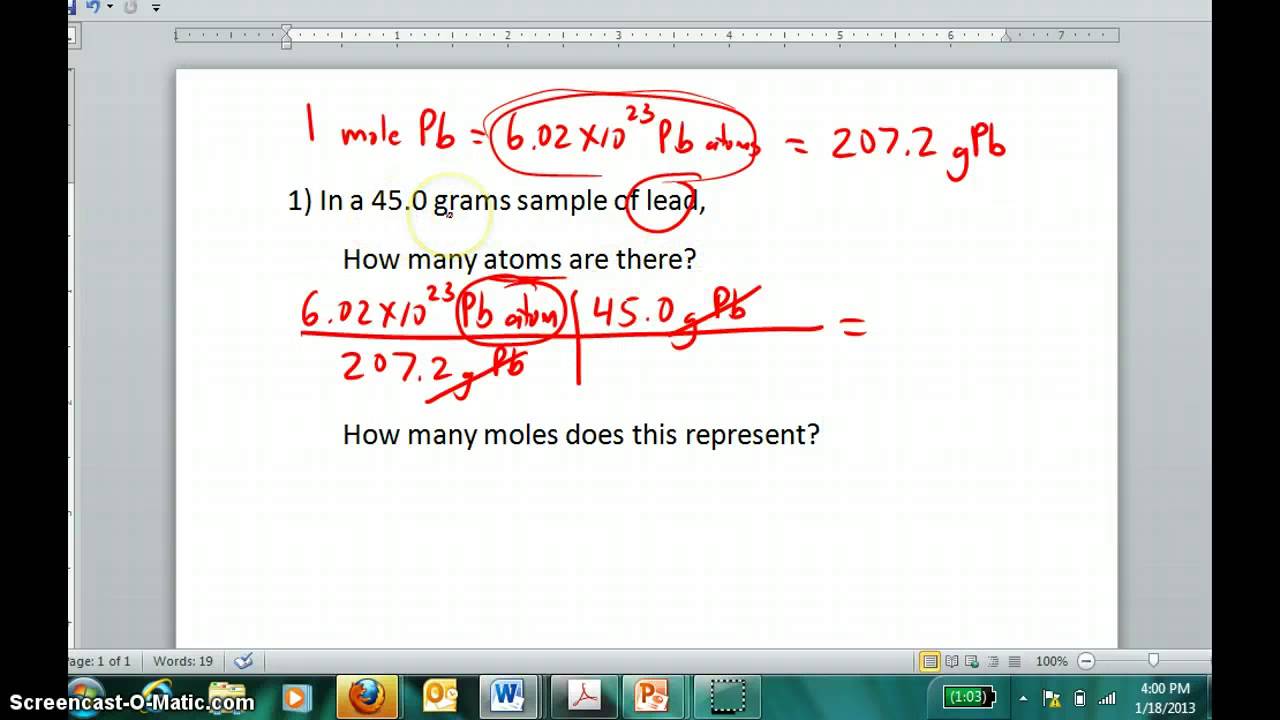 Source: pinterest.com
Source: pinterest.com
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382. One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. How To Calculate The Quantity Of Moles The Method For The Quantity Of Moles Is Mass Divided By Molar Mass Instructing Chemistry Chemistry Classroom Science For Youngsters. Experts are tested by Chegg as specialists in their subject area.
 Source: pinterest.com
Source: pinterest.com
Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382. Thus the number of moles in the given sample of. Numerically this would be 2 1008 1 1600 18016. Calculating The Moles Of An Aspect In A Compound Youtube. Next multiply the atomic mass of each atom by the number of atoms in the compound.
 Source: pinterest.com
Source: pinterest.com
This is the molar mass of the compound. Number of moles formula is. Who are the experts. There are three hydrogen atoms as indicated by the subscript. Popular Answers 1 Calculate he number of moles you have by taking the Mass molar mass.
 Source: pinterest.com
Source: pinterest.com
9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. This chemistry video tutorial explains how to calculate the molar mass of a compound. Mass of one mole MnO2 8694g. This will give you the relative amount that each element contributes to the. Mass of MnO2 95g.
 Source: pinterest.com
Source: pinterest.com
It contains plenty of examples and practice problemsMy E-Book. For example one molecule of H2O has two atoms of Hydrogen H. This is the molar mass of the compound. Repeat steps 5-7 for the other compounds and record your. In our example the weight of NaCl is 100 grams and its molecular weight is 5852 gmoles.
 Source: pinterest.com
Source: pinterest.com
Experts are tested by Chegg as specialists in their subject area. Calculate the moles of aspirin. Multiply the elements atomic mass by the number of atoms of that element in the compound. Calculate the atoms of each element in C 12 H 22 O 11 and record your results in Table 2. Divide the mass of the compound in grams by the molar mass you just calculated.
 Source: pinterest.com
Source: pinterest.com
Molarity is the concentration of a solution expressed as the number of moles of solute per litre of solution How do you identify an unknown acid. Repeat steps 5-7 for the other compounds and record your. 325 mg C 6 H 4 CO 2 H CO 2 CH 3 10 - 3 g 1 mg 1 mole C 6 H 4 CO 2 H CO 2 CH 3 180. To convert between grams and moles you would use the substances molar mass. Calculating The Moles Of An Aspect In A Compound Youtube.
 Source: pinterest.com
Source: pinterest.com
It has units of grams per mole. This will give you the relative amount that each element contributes to the. If you have 1000 grams. It has units of grams per mole. How To Calculate The Quantity Of Moles The Method For The Quantity Of Moles Is Mass Divided By Molar Mass Instructing Chemistry Chemistry Classroom Science For Youngsters.
 Source: pinterest.com
Source: pinterest.com
Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382. Numerically this would be 2 1008 1 1600 18016. If the titrant and analyte have a 11 mole ratio the formula is molarity M of the acid x volume V of the acid molarity M of the base x volume V of the base. If you have 1000 grams. Then 1000 g 151001 gmol.
 Source: pinterest.com
Source: pinterest.com
How do you find the moles of an element in a compound. The formula for moles to grams is given by. Then 1000 g 151001 gmol. Next multiply the atomic mass of each atom by the number of atoms in the compound. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to determine moles of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






