Your How to determine moles from molar mass images are ready. How to determine moles from molar mass are a topic that is being searched for and liked by netizens today. You can Find and Download the How to determine moles from molar mass files here. Find and Download all royalty-free images.
If you’re looking for how to determine moles from molar mass images information linked to the how to determine moles from molar mass interest, you have pay a visit to the ideal site. Our site always provides you with suggestions for seeking the maximum quality video and picture content, please kindly surf and find more enlightening video articles and images that match your interests.
How To Determine Moles From Molar Mass. The molar mass is 9757 gmol. 100 mL 1082 g1 mL 108 g 2. From Boiling Point Elevation. In gas calculations there are conditions known as STP - Standard Temperature and Pressure.
 How To Calculate The Number Of Moles From surfguppy.com
How To Calculate The Number Of Moles From surfguppy.com
Divide the mass by the molar mass to get the number of moles. Then from the Molality of the compound moles of A per kg of B M mass of A in the compound molar mass of A per kg B. We can use a measurement of any one of the following properties to determine the molar mass molecular weight of an unknown that is the solute in a solution. Sep 21 2017 Mathematically the defining equation of molar mass is Molar mass massmole gmol The definition of atomic mass the mole and molar mass are all immediately or not directly. Temperature 0C or 273156K and absolute temperature in K h. So the molar mass of glucose is going to be six times the molar mass of carbon plus 12 times the molar mass of hydrogen plus six times the molar mass of oxygen.
Here you are once.
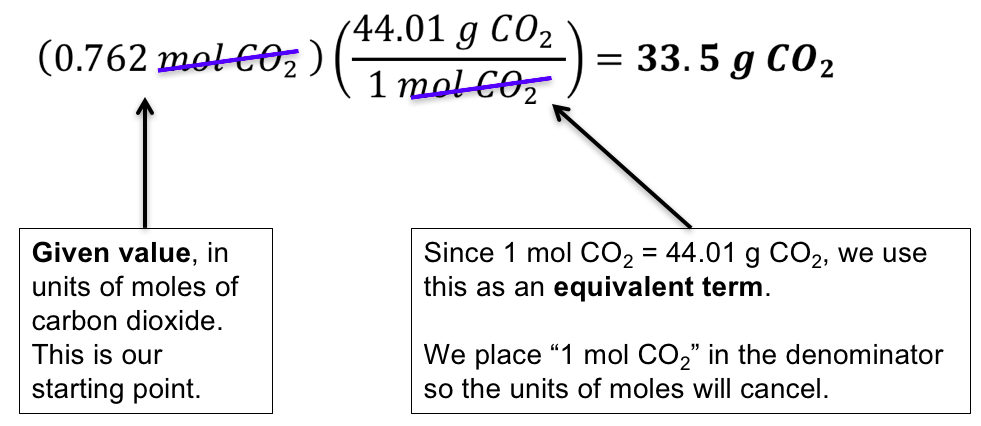
Thus by knowing the molar mass we can determine the number of moles contained in a given mass of a sample. And so now we have all the information we need from our periodic table of elements. Then 108 g 1 mol1021 g 0106 mol. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. The grams of acid are determined from weighing the acid and the moles are determined from the titration with NaOH. Moles mol Molarity M x Volume L 05 x 2.
 Source: youtube.com
Source: youtube.com
From Boiling Point Elevation. Converting Grams to Moles Using Molar Mass How to Pass Chemistry - YouTube. How do you find molar mass from molarity. Answer 1 of 4. For NaCl the molar mass is 5844 gmol.
 Source: chem.fsu.edu
Source: chem.fsu.edu
M molar mass of pure substance in grams per mole. In gas calculations there are conditions known as STP - Standard Temperature and Pressure. Ask me questions on Facebook. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. The molar mass is 9757 gmol.
 Source: nagwa.com
Source: nagwa.com
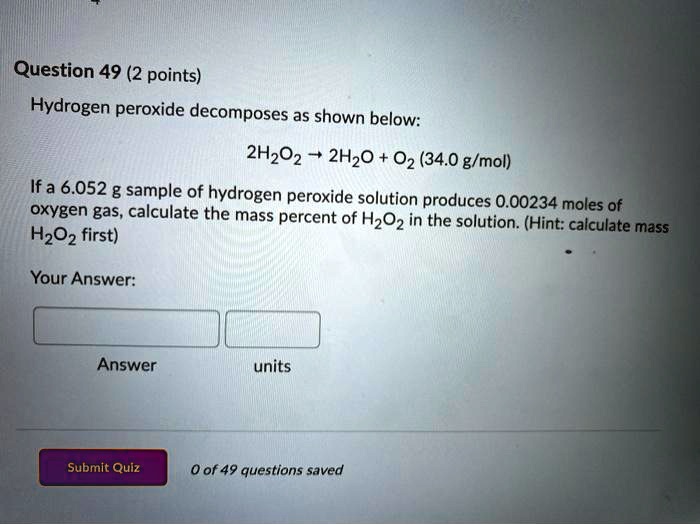
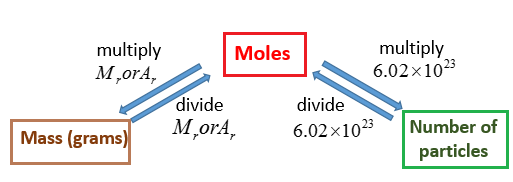
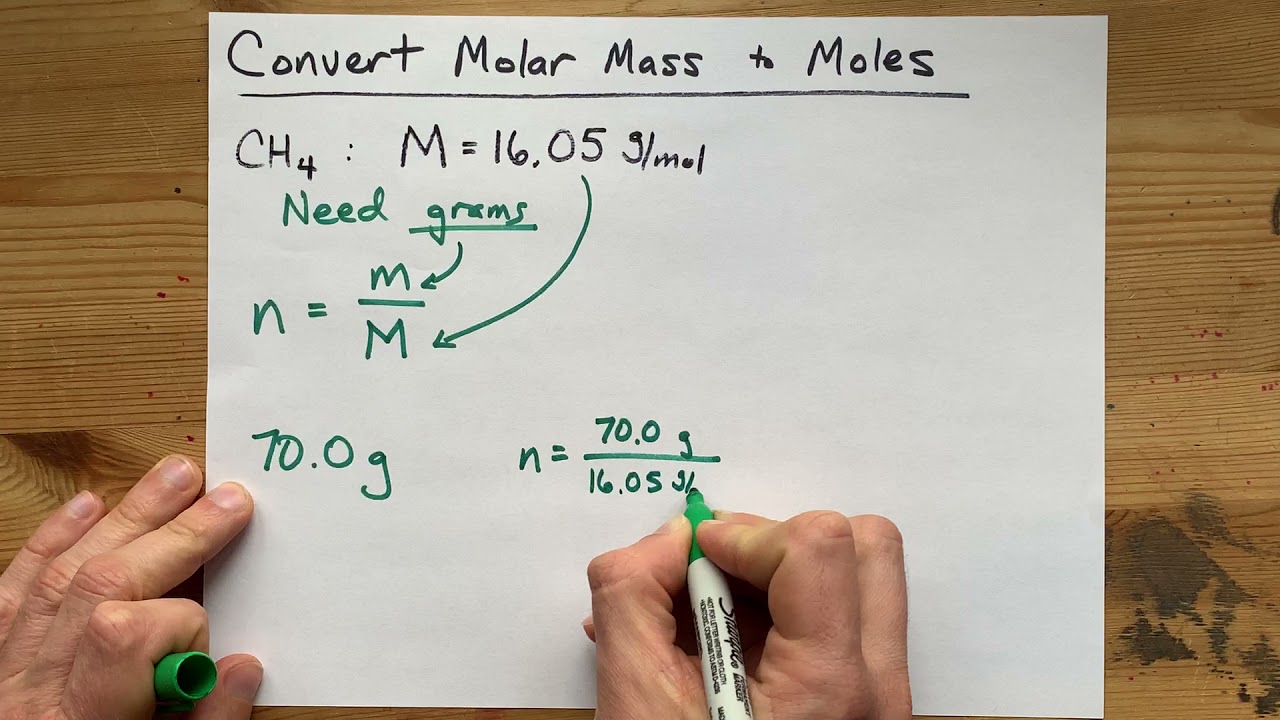
Mass moles mass of 1 mole. Therefore the units of molar mass are gramsmoleHow to find the molar mass of a compound. From you calculate the molar mass to be 1021 gmol. Then 108 g 1 mol1021 g 0106 mol. Number of moles mass relative formula mass This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known.
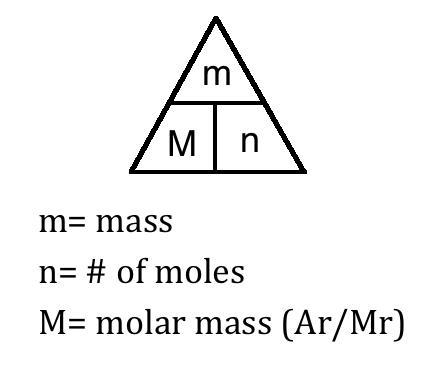 Source: derekcarrsavvy-chemist.blogspot.com
Source: derekcarrsavvy-chemist.blogspot.com
The formula for density is. Now we can use the rearranged equation. Converting Grams to Moles Using Molar Mass How to Pass Chemistry - YouTube. 100 mL 1082 g1 mL 108 g 2. Moles mol x Molar Mass gmol 1 x 5844.
 Source: surfguppy.com
Source: surfguppy.com
Multiply the atomic weight of each element with its number of atoms present in the compound. First you must calculate the number of moles in this solution by rearranging the equation. Ask me questions on Facebook. Molar mass 558 gmol. Now we can use the rearranged equation.
 Source: studylib.net
Source: studylib.net
The molar mass links the mass of a substance to its moles. MOLES FROM VOLUME OF PURE LIQUID OR SOLID. Multiply the atomic weight of each element with its number of atoms present in the compound. How do you find molar mass from molarity. Moles mol Molarity M x Volume L 05 x 2.
 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
Let A be the solute and molar mass of A be Ma. Therefore the units of molar mass are gramsmoleHow to find the molar mass of a compound. Also the periodic table can help you to find the molar mass of each element. The molar mass of acetic anhydride is 1021 gmol and its density is 1080 gmL. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg.
 Source: clutchprep.com
Source: clutchprep.com
For finding out this you have to multiply the mass of solute by its molar mass conversion factor. Oxygen we can see from our periodic table of elements it has a molar mass of 1600 grams per mole. So its going to be six times 1201. Mass g No. There are two steps.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Now we can use the rearranged equation. Answer 1 of 4. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. How do you find molar mass from molarity. Oxygen we can see from our periodic table of elements it has a molar mass of 1600 grams per mole.
 Source: khanacademy.org
Source: khanacademy.org
Convert grams to moles. Most noteworthy each molecule has 1 Na Sodium and 1 Cl. The grams of acid are determined from weighing the acid and the moles are determined from the titration with NaOH. M molar mass of pure substance in grams per mole. MOLES FROM VOLUME OF PURE LIQUID OR SOLID.
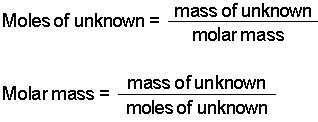 Source: chem.purdue.edu
Source: chem.purdue.edu
100 mL 1082 g1 mL 108 g 2. Moles mmn calculate the molar mass of a pure substance if 175 moles of the substance has a mass of 2979 g. Moles mol x Molar Mass gmol 1 x 5844. Multiply the volume by the density to get the mass. The grams of acid are determined from weighing the acid and the moles are determined from the titration with NaOH.
 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
Mass g No. The formula for density is. We can use a measurement of any one of the following properties to determine the molar mass molecular weight of an unknown that is the solute in a solution. Mass moles mass of 1 mole. How many moles are in 1000 mL of acetic anhydride.
 Source: youtube.com
Source: youtube.com
There are two steps. Ask me questions on Facebook. The screenshot below displays the page or activity to enter your values to get the answer for the molar mass according to the respective parameters which are the Mass m and Number of moles n. Convert millilitres to grams. Number of moles mass relative formula mass This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known.
 Source: chemistryvce.weebly.com
Source: chemistryvce.weebly.com
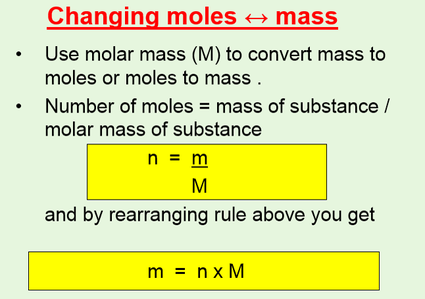
Then from the Molality of the compound moles of A per kg of B M mass of A in the compound molar mass of A per kg B. It is good to memorize that moles gramsmolar mass. Thus by knowing the molar mass we can determine the number of moles contained in a given mass of a sample. MOLES FROM VOLUME OF PURE LIQUID OR SOLID. And since mass of 1 mole of a substance in grams molar mass in grams per mole mass g moles molar mass g mol -1 m n M.
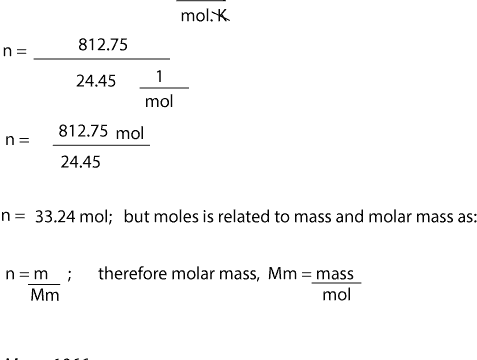 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
Then from the Molality of the compound moles of A per kg of B M mass of A in the compound molar mass of A per kg B. The grams of acid are determined from weighing the acid and the moles are determined from the titration with NaOH. Add up all and assign unit as gramsmole. Converting Grams to Moles Using Molar Mass How to Pass Chemistry - YouTube. Number of moles mass relative formula mass This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to determine moles from molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.