Your How to determine mole ratio of a compound images are available. How to determine mole ratio of a compound are a topic that is being searched for and liked by netizens today. You can Find and Download the How to determine mole ratio of a compound files here. Download all royalty-free images.
If you’re searching for how to determine mole ratio of a compound pictures information related to the how to determine mole ratio of a compound topic, you have visit the ideal site. Our site frequently provides you with hints for viewing the maximum quality video and picture content, please kindly search and find more informative video articles and graphics that match your interests.
How To Determine Mole Ratio Of A Compound. Choose a signal and calculates the area of H separately of each compound. Multiply the molar mass of each element by its subscript from the molecular formula. Then draw a horizontal isotherm at that point red line on the diagram above. Find the molar mass of each element on the periodic table.
 The Math Of Chemical Reactions Ppt Download From slideplayer.com
The Math Of Chemical Reactions Ppt Download From slideplayer.com
In other words 1 mol of methane will produced 1 mole of carbon dioxide as long as the reaction goes to completion and there is plenty of oxygen present. How many moles are in a liter. If you want to calculate the mole ratio as a prove you can use the mass weight ratio or atomic ratio. Find the molar mass of each element on the periodic table. A mole ratio is the ratio between the amounts in moles of any two compounds involved in a chemical reaction. Calculate the molar ratios.
To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant.
We identified it from well-behaved source. Divide the mass of anhydrate by the molar mass of anhydrate to get moles of anhydrate. Also determine the water in the hydrate. Then draw a horizontal isotherm at that point red line on the diagram above. To find the simplest whole number ratio divide each number by the smallest number of moles. The ratio between them is the molar ratio.
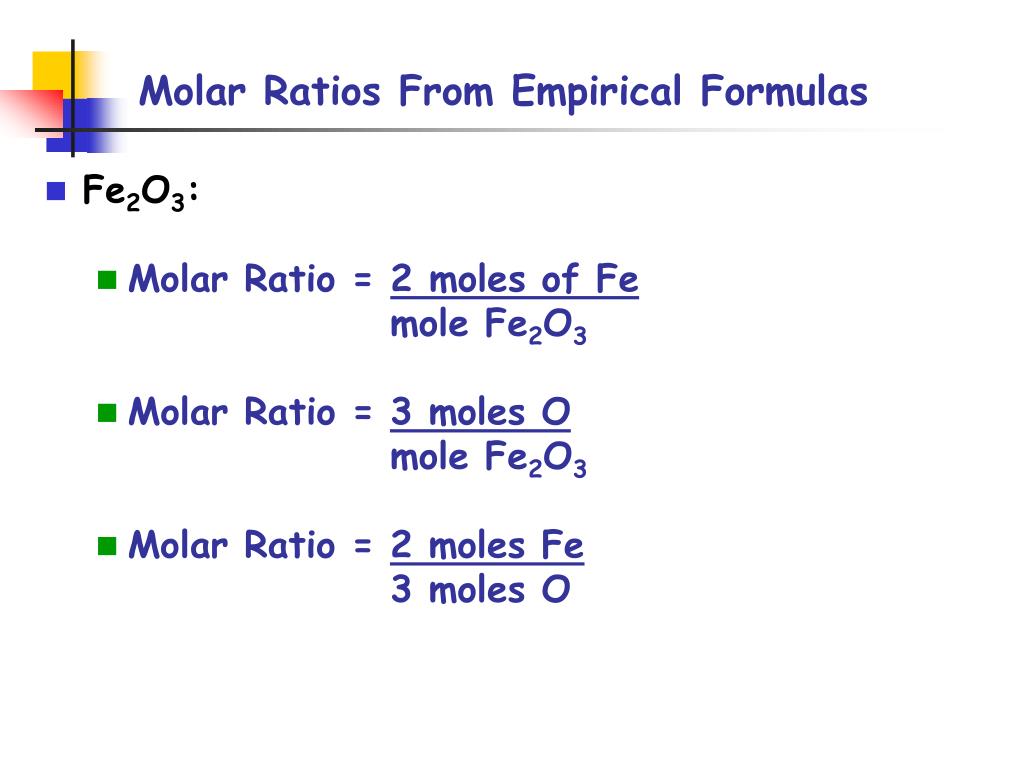 Source: slideserve.com
Source: slideserve.com
Find the number of atoms of. Round off the mole ratio to the nearest whole number if less than 019 or greater than 081 fractions. Multiply the molar mass of each element by its subscript from the molecular formula. Round to the nearest whole number. This is the mole ratio of the elements and is.
 Source: slidetodoc.com
Source: slidetodoc.com
To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. Draw a line vertically from that composition to the bubble point or liquid line. To calculate the molar ratios you set the moles of 1 reactant over the moles of the opposite reactant. To find the simplest whole number ratio divide each number by the smallest number of moles. 2 mol H 2 O.
 Source: youtube.com
Source: youtube.com
Multiply the molar mass of each element by its subscript from the molecular formula. Round off the mole ratio to the nearest whole number if less than 019 or greater than 081 fractions. A mole ratio is the ratio between the amounts in moles of any two compounds involved in a chemical reaction. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. The equality of 1 mole 224 L is the basis for the conversion factor.
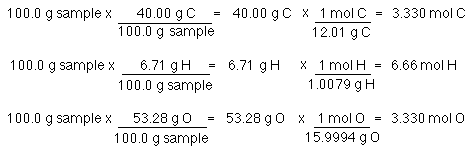 Source: westfield.ma.edu
Source: westfield.ma.edu
Choose a signal and calculates the area of H separately of each compound. Then draw a horizontal isotherm at that point red line on the diagram above. The numbers of moles of each element are in the same ratio as the number of atoms C H and O in vitamin C. The mole ratio can be known as the mole-to. Multiply the molar mass of each element by its subscript from the molecular formula.
 Source: slideplayer.com
Source: slideplayer.com
For each one mole of CuSO 4 there are 5 moles of water. Otherwise multiply the mole ratio by a number which gives two whole numbers for the ratio within 01 range of the whole number. The numbers of moles of each element are in the same ratio as the number of atoms C H and O in vitamin C. Remember hydrates have a fixed amount of water per mole of the non-water compound. Mole ratios are used as conversion factors between products and reactants in many chemistry problems.
 Source: quizlet.com
Source: quizlet.com
Figuring out Empirical Formulation Worksheet College Assist How To Discover Out Chemistry. In this example the molar ratio is 15. Convert all masses into moles. Moles H 458 g H x 1 mol H 101 g H 453 mol H. For every one mole of CuSO 4 there are five moles of water.
 Source: pinterest.com
Source: pinterest.com
Then draw a horizontal isotherm at that point red line on the diagram above. For every one mole of CuSO 4 there are five moles of water. Identify the Mole Ratio of the given compound. Here are a number of highest rated How To Calculate Mole Ratio pictures upon internet. In this example the smallest height is 17 mm so you divide both heights by 17 mm.
 Source: dummies.com
Source: dummies.com
Choose a signal and calculates the area of H separately of each compound. In other words 1 mol of methane will produced 1 mole of carbon dioxide as long as the reaction goes to completion and there is plenty of oxygen present. 240g I₂ 1 mol I2 2538g I₂ 0009 456 mol I2. A mole ratio is the ratio between the amounts in moles of any two compounds involved in a chemical reaction. To determine the the empirical formula for molar mass.
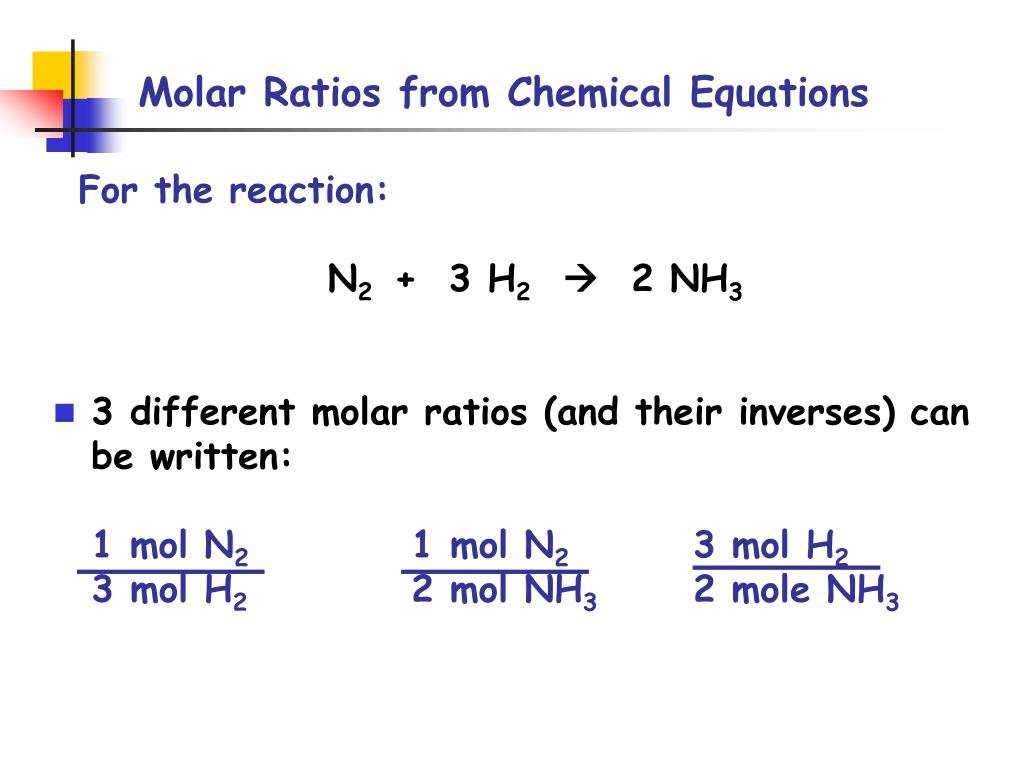 Source: slideserve.com
Source: slideserve.com
The equality of 1 mole 224 L is the basis for the conversion factor. Round to the nearest whole number. Find the sum of these products for all the elements in the formula. Moles H 458 g H x 1 mol H 101 g H 453 mol H. Draw a line vertically from that composition to the bubble point or liquid line.
 Source: nagwa.com
Source: nagwa.com
A 25 to 1 is really a 5 to 2 ratio. Mole ratio of methane. How many moles are in a liter. Remember hydrates have a fixed amount of water per mole of the non-water compound. Here are a number of highest rated How To Calculate Mole Ratio pictures upon internet.
 Source: bulbapp.com
Source: bulbapp.com
Find the sum of these products for all the elements in the formula. CarbonHydrogen 14 Step 2. 240g I₂ 1 mol I2 2538g I₂ 0009 456 mol I2. Otherwise multiply the mole ratio by a number which gives two whole numbers for the ratio within 01 range of the whole number. In this example the smallest height is 17 mm so you divide both heights by 17 mm.
 Source: nagwa.com
Source: nagwa.com
If you want to calculate the mole ratio as a prove you can use the mass weight ratio or atomic ratio. For every one mole of CuSO 4 there are five moles of water. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. Notice that this is not a chemical reaction it is simply a chemical formula of a hydrate. Convert all masses into moles.
 Source: youtube.com
Source: youtube.com
These molar ratios can also be expressed as fractions. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. How do you find the ratio of an element in a compound. 1 mol I2 2538g I₂ 0009 456 mol I2. Mass percent grams of solute grams of solute plus solvent x 100.
 Source: slideplayer.com
Source: slideplayer.com
CarbonHydrogen 14 Step 2. A 25 to 1 is really a 5 to 2 ratio. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. Feb 09 2021 Steps to find out empirical system. These molar ratios can also be expressed as fractions.
 Source: youtube.com
Source: youtube.com
A mole ratio is the ratio between the amounts in moles of any two compounds involved in a chemical reaction. To determine the the empirical formula for molar mass. 120g Al 1 mol Al 2698g Al 0044 48 mol Al. 240g I₂ 1 mol I2 2538g I₂ 0009 456 mol I2. Also determine the water in the hydrate.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to determine mole ratio of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






