Your How to determine grams per mole images are available. How to determine grams per mole are a topic that is being searched for and liked by netizens today. You can Get the How to determine grams per mole files here. Download all free photos.
If you’re looking for how to determine grams per mole images information connected with to the how to determine grams per mole topic, you have pay a visit to the right site. Our website always gives you suggestions for viewing the highest quality video and picture content, please kindly hunt and find more informative video articles and graphics that fit your interests.
How To Determine Grams Per Mole. This converts it to grams per 1000 mL or better yet grams per liter. We use molar mass which is grams per mole. You can find these using the periodic table. 1 mole 746 g 3 grams 3 746 0040 moles Express this as moles per kilogram solution.
 Mole Gram Formula Mass Moles To Grams And Grams To Moles Ppt Download From slideplayer.com
Mole Gram Formula Mass Moles To Grams And Grams To Moles Ppt Download From slideplayer.com
Hence one mole of H2SO4 weights 106076 grams. Therefore for 16 grams of water there is 089 moles. And 1 mol of chlorine Cl has a mass of 35453 g the mass on the periodic table. Example 1 Calculate the mass in grams of 36 mol of H2SO4. In grams a mole is one formula mass. Thus the number of moles in the given sample of.
1 multiply the g100mL value by 1010.
How to convert grams to moles. For NaCl the molar mass is 5844 gmol. Thus the number of moles in the given sample of. More free chemistry help videos. If you know the quantity of mole it can be converted into grams and vice versa. First you must calculate the number of moles in this solution by rearranging the equation.
 Source: youtube.com
Source: youtube.com
If you had the compound sodium. Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³. What do we multiply moleslitre by to get gramslitre. 0700 mole x 340146 gramsmole 238 grams. Calculate the moles from the grams.
 Source: khanacademy.org
Source: khanacademy.org
This converts it to grams per 1000 mL or better yet grams per liter. You may wish to pause and calculate this value if you desire the practice. If you have gdm3 and you divide by the molar mass molg the grams will cancel out and youll be left with moldm3. To convert from grams to moles you need to use the molar mass. One mole consists of Avogadro number of atoms.
 Source: wikihow.com
Source: wikihow.com
This is the number of atoms in each element that makes up the compound. 1 mole 746 g 3 grams 3 746 0040 moles Express this as moles per kilogram solution. This is the number of atoms in each element that makes up the compound. Calculating the Molar Mass of a Compound. More free chemistry help videos.
 Source: wikihow.com
Source: wikihow.com
Definition of Number of Moles. Mass g No. If you have gdm3 and you divide by the molar mass molg the grams will cancel out and youll be left with moldm3. It is the amount of grams that is numerically equal to the molecular weight. Thus the number of moles in the given sample of.
 Source: wikihow.com
Source: wikihow.com
When that happens this step is skipped 2 divide the grams per liter value by the molar mass of the substance. For NaCl the molar mass is 5844 gmol. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988. One mole consists of Avogadro number of atoms. For a molecule with a weight of 18 the molar mass is 18 grams.
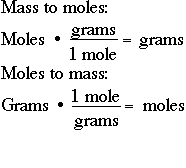 Source: socratic.org
Source: socratic.org
You can find these using the periodic table. The molar mass of a substance is calculated by adding together the molar mass of the components involved. How do you convert molarity to grams per mole. The number 6022140761023 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons. You may wish to pause and calculate this value if you desire the practice.
 Source: khanacademy.org
Source: khanacademy.org
Since water has two molecules of hydrogen and one molecule of oxygen then the molecular weight of water is 1801528gmol. Substance in moles grams and concentration of substance in moll gl For conversion from mass to molarity divide the mass g or gl with molar mass relative AWMWFW For conversion from molarity to mass multiply the molarity mol or moll with molar mass relative AWMWFW Concentration of a solution in. Mass g No. If you have gdm3 and you divide by the molar mass molg the grams will cancel out and youll be left with moldm3. In our example the weight of NaCl is 100 grams and its molecular weight is 5852 gmoles.
 Source: youtube.com
Source: youtube.com
How many grams Cm in 1 mol. Now you have 250 ml of water which is about 250 g of water assuming a density of 1 gml but you also have 3 grams of solute so the total mass of the solution is closer to 253 grams than 250. It can be expressed as grams liters atoms molecules or particles. Convert the volume of the water to its mass assuming that the density of pure water is 998 kgm³. In grams a mole is one formula mass.
 Source: slideplayer.com
Source: slideplayer.com
For example for NaCl you would need the molar mass of sodium and the molar mass of chloride. You may wish to pause and calculate this value if you desire the practice. Now we are already in litres so how to convert moles to grams. It can be expressed as grams liters atoms molecules or particles. For a molecule with a weight of 32 th.
 Source: youtube.com
Source: youtube.com
This converts it to grams per 1000 mL or better yet grams per liter. Example 1 Calculate the mass in grams of 36 mol of H2SO4. For a molecule with a weight of 32 th. How to convert grams to moles. The number 6022140761023 the Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal for most practical purposes to the average mass of one molecule of the compound in daltons.
 Source: discoveryexpresskids.com
Source: discoveryexpresskids.com
If you have gdm3 and you divide by the molar mass molg the grams will cancel out and youll be left with moldm3. A mole can be defined as the amount of substance. How to convert grams to moles. Molarity x molar mass moleslitre x grams mole the moles cancel leaving. How many grams Cm in 1 mol.
 Source: youtube.com
Source: youtube.com
0700 mole x 340146 gramsmole 238 grams. Definition of Number of Moles. Multiply the moles given by the substances molar mass. To convert from grams to moles you need to use the molar mass. First you must calculate the number of moles in this solution by rearranging the equation.
 Source: slideplayer.com
Source: slideplayer.com
Calculate the moles from the grams. When that happens this step is skipped 2 divide the grams per liter value by the molar mass of the substance. Find the molar mass of a water molecule H₂O. For a molecule with a weight of 32 th. Now you have 250 ml of water which is about 250 g of water assuming a density of 1 gml but you also have 3 grams of solute so the total mass of the solution is closer to 253 grams than 250.
 Source: study.com
Source: study.com
You may wish to pause and calculate this value if you desire the practice. Sowe want to end up with grams litre from moleslitre. Now we are already in litres so how to convert moles to grams. Moles mol Molarity M x Volume L 05 x 2. The molar mass is the mass of one mole of a substance.

So for every one mole of glucose C6H12O6 we have 18016 grams of glucose C6H12O6 and this is going to get us we get 152 times 1000 is equal to this is the number of grams of glucose we have and then were going to divide by 18016 divide by 18016 gives us this number and lets see if we see significant figures we have three significant figures here we have five here so. For a molecule with a weight of 32 th. How do you convert molarity to grams per mole. Accordingly how do you convert moles to dm3. 1 multiply the g100mL value by 1010.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to determine grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.