Your How to convert moles to grams steps images are ready in this website. How to convert moles to grams steps are a topic that is being searched for and liked by netizens now. You can Get the How to convert moles to grams steps files here. Find and Download all free photos and vectors.
If you’re searching for how to convert moles to grams steps images information linked to the how to convert moles to grams steps keyword, you have visit the ideal blog. Our website frequently gives you hints for viewing the maximum quality video and image content, please kindly surf and locate more informative video content and graphics that fit your interests.
How To Convert Moles To Grams Steps. M 3899 107868. Using the factor 9808 g 1 mol. When you use your kitchen scale you may get the amount of the substance in grams ounces or. In other words it is the product of the mass of the substance and its molecular weight.
 How To Convert Between Molecules Moles And Grams Examples Practice Problems Summary Youtube From youtube.com
How To Convert Between Molecules Moles And Grams Examples Practice Problems Summary Youtube From youtube.com
Now we have to perform moles to grams calculation. M 420577332 g. Thus one mole of H 2 SO 4 weighs 9808 grams. These measurements are used to determine the number of substances. Of moles of HCl Mass of HCl 1365 365 grams. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question.
Of moles of HCl Mass of HCl 1365 365 grams.
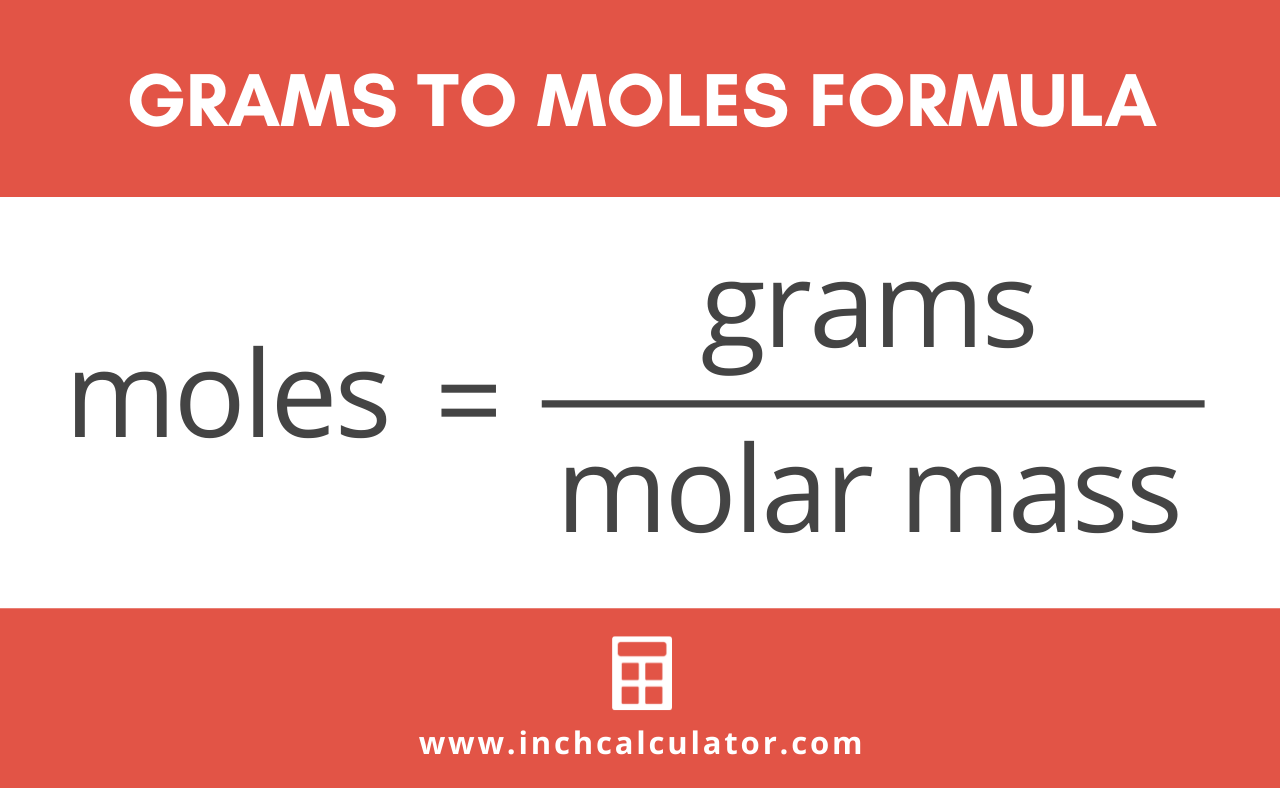
Here are three important steps to follow to convert moles to grams. Divide the number of grams of the substance by the molecular mass. Here are three important steps to follow to convert moles to grams. How to Convert Grams to Moles of Carbon Dioxide When the Given Requirement is 45 Moles of Carbon Dioxide. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Thus for hydrochloric acid 1 mole is equal to 365 grams of HCl.
 Source: uctsc.org
Source: uctsc.org
Thus for hydrochloric acid 1 mole is equal to 365 grams of HCl. Divide the number of grams of the substance by the molecular mass. Convert 02 moles of Sodium chloride. Thus for hydrochloric acid 1 mole is equal to 365 grams of HCl. One mole consists of Avogadro number of atoms.
 Source: slideplayer.com
Source: slideplayer.com
To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Calculate how many moles are mentioned in the question. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Find the molar mass of the substance. Of moles of HCl Mass of HCl 1365 365 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
Calculate how many moles are mentioned in the question. You have three steps to convert mole values to grams. There are 353 grams of H 2 SO 4 in 360 moles of H 2 SO 4. Now calculate the molar mass of the substance. You can also verify the results by fetching the.
 Source: uctsc.org
Source: uctsc.org
M 420577332 g. In other words it is the product of the mass of the substance and its molecular weight. Thus one mole of H 2 SO 4 weighs 9808 grams. Divide the number of grams of the compound by its molecular mass. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question.
 Source: uctsc.org
Source: uctsc.org
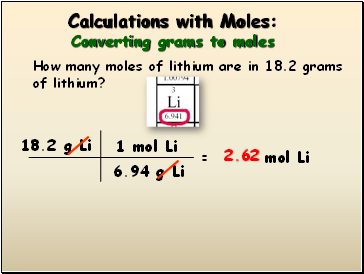
In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question. You can also verify the results by fetching the. After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive. Grams H 2 SO 4 360 mol x 9808 g 1 mol 353 g H 2 SO 4. There are 353 grams of H 2 SO 4 in 360 moles of H 2 SO 4.
 Source: youtube.com
Source: youtube.com
M 3899 107868. Thus one mole of H 2 SO 4 weighs 9808 grams. Grams Moles x Molar Mass. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions.
 Source: uctsc.org
Source: uctsc.org
You can also verify the results by fetching the. Thus for hydrochloric acid 1 mole is equal to 365 grams of HCl. Therefore the molecular weight in grams of 1 mole of HCl No. The answer will be the number of moles of the compound. M 420577332 g.
 Source: uctsc.org
Source: uctsc.org
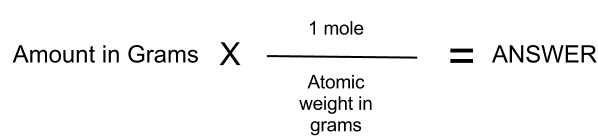
Determine how many moles are given in the problem. Using the factor 9808 g 1 mol. Multiply both the values. Grams Molar Mass of substance Given Moles. Calculate the molar mass of the substance.
 Source: uctsc.org
Source: uctsc.org
Convert 02 moles of Sodium chloride. Now we have to perform moles to grams calculation. One mole consists of Avogadro number of atoms. In other words it is the product of the mass of the substance and its molecular weight. Divide the number of grams of the compound by its molecular mass.
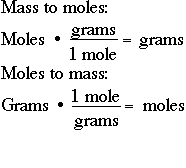 Source: socratic.org
Source: socratic.org
When you use your kitchen scale you may get the amount of the substance in grams ounces or. M 3899 107868. When you use your kitchen scale you may get the amount of the substance in grams ounces or. I n Chemistry the moles to grams conversion represents the conversion of moles into grams. Using the factor 9808 g 1 mol.
 Source: youtube.com
Source: youtube.com
One mole consists of Avogadro number of atoms. To convert the moles into grams multiply the mass of the substance by the molecular weight formula weight. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. If you know the quantity of mole it can be converted into grams and vice versa. 02 x 5844 11688 grams.
 Source: youtube.com
Source: youtube.com
Calculate how many moles are mentioned in the question. When you use your kitchen scale you may get the amount of the substance in grams ounces or. This relation provides a conversion factor to go from grams to moles. In the last step you should multiply both the values. If you know the quantity of mole it can be converted into grams and vice versa.
 Source: uctsc.org
Source: uctsc.org
I n Chemistry the moles to grams conversion represents the conversion of moles into grams. You have three steps to convert mole values to grams. Converting grams to moles involves 2 steps. In the last step you should multiply both the values. For calculations tap Molar Mass Calculator By using moles to grams formula.
 Source: study.com
Source: study.com
Formula to convert moles to grams. How to Convert Grams to Moles. Using the factor 9808 g 1 mol. For calculations tap Molar Mass Calculator By using moles to grams formula. In other words it is the product of the mass of the substance and its molecular weight.
 Source: uctsc.org
Source: uctsc.org
When you use your kitchen scale you may get the amount of the substance in grams ounces or. Multiply both the values. Thus one mole of H 2 SO 4 weighs 9808 grams. You can also verify the results by fetching the. There are 353 grams of H 2 SO 4 in 360 moles of H 2 SO 4.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to convert moles to grams steps by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






