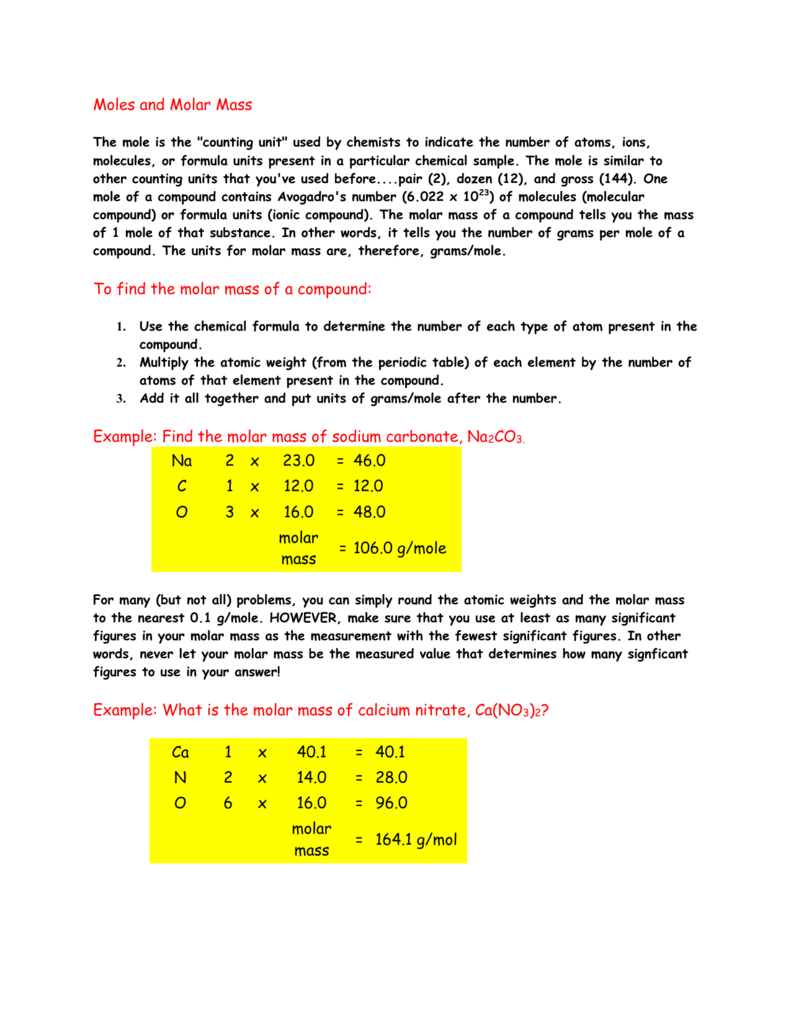
Your How to convert moles of a compound to grams images are ready. How to convert moles of a compound to grams are a topic that is being searched for and liked by netizens now. You can Find and Download the How to convert moles of a compound to grams files here. Download all free images.
If you’re looking for how to convert moles of a compound to grams images information linked to the how to convert moles of a compound to grams topic, you have come to the ideal blog. Our website always provides you with suggestions for downloading the highest quality video and image content, please kindly search and find more informative video articles and graphics that match your interests.
How To Convert Moles Of A Compound To Grams. 0700 mole x 340146 gramsmole 238 grams the answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures. Number of moles Weight of compound in grams molecular weight of compound In our example the weight of NaCl is 100 grams and its molecular weight is 5852 gmoles. Convert another chemical substance Convert moles to grams Quick conversion chart of moles Br to grams 1 moles Br to grams 79904 grams 2 moles Br to grams 159808 grams 3 moles Br to grams 239712 grams 4 moles Br to grams 319616 grams 5 moles Br to grams 39952 grams. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems.
 Ruthlearns Chemistry Is Badass Chemistry Education Physical Science High School Chemistry From pinterest.com
Ruthlearns Chemistry Is Badass Chemistry Education Physical Science High School Chemistry From pinterest.com
In case you have x grams you devide x by grams per mole to get moles. Note that rounding errors may occur so always check the results. Divide the initial mass of the. The formula for moles to grams is given by Example 1 Calculate the mass in grams of 36 mol of H2SO4. Additionally how many moles are in a gram. Lets figure out what the difference between molar mass and atomic mass is and learn to use molar mass as a conversion factor and stop guessing on how to con.
You can view more details on each measurement unit.
The formula mass of H 2 SO 4 is. How many moles Ag in 1 grams. To convert grams to moles. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Take the number of moles and multiply them by Avogadros number. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
We assume you are converting between moles HgO and gram. Convert another chemical substance Convert moles to grams Quick conversion chart of moles Br to grams 1 moles Br to grams 79904 grams 2 moles Br to grams 159808 grams 3 moles Br to grams 239712 grams 4 moles Br to grams 319616 grams 5 moles Br to grams 39952 grams. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. A common request on this site is to convert grams to moles. Quick Reference Guide For Calculating And Converting Moles.
 Source: pinterest.com
Source: pinterest.com
Solution Look for the atomic masses of hydrogen sulfur and oxygen. Convert another chemical substance Convert moles to grams Quick conversion chart of moles Br to grams 1 moles Br to grams 79904 grams 2 moles Br to grams 159808 grams 3 moles Br to grams 239712 grams 4 moles Br to grams 319616 grams 5 moles Br to grams 39952 grams. The formula for moles to grams is given by Example 1 Calculate the mass in grams of 36 mol of H2SO4. Apr 15 2020 To transform moles into grams decide the variety of moles preset and the molar mass of the compound. Similarly we evaluate the moles using the compounds mass and considering its.
 Source: pinterest.com
Source: pinterest.com
You can carry out moles to grams conversion with the help of the following equation below. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. To convert grams to moles. How to go from moles to grams. Once you have both the molecular mass and physical weight of the compound you can use a simple formula to calculate the number of moles present in the sample.
 Source: pinterest.com
Source: pinterest.com
Moles To Grams Formula. Quick Reference Guide For Calculating And Converting Moles. The atomic mass is 1008 for H 3206 for S and 1600 for O. It is calculated by measuring the molecular weight of the chemical compound which tells us how many grams are in one mole of that substance. 7 moles in to grams 803726 gramsBased on that information to convert 1009 moles of curium to grams we multiply 1009 moles of curium by 247.
 Source: pinterest.com
Source: pinterest.com
The formula mass of H 2 SO 4 is. You can view more details on each measurement unit. This compound is also known as Mercury II Oxide. We assume you are converting between moles HgO and gram. To convert moles to particles atoms or molecules.
 Source: pinterest.com
Source: pinterest.com
Divide the initial mass of the. You can view more details on each measurement unit. Once you have both the molecular mass and physical weight of the compound you can use a simple formula to calculate the number of moles present in the sample. Solution First look up the atomic masses for hydrogen sulfur and oxygen from the periodic table. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions.
 Source: pinterest.com
Source: pinterest.com
Lets figure out what the difference between molar mass and atomic mass is and learn to use molar mass as a conversion factor and stop guessing on how to con. 2 1008 3206 4 1600 9808 Thus one mole of H 2 SO 4 weighs 9808 grams. You can carry out moles to grams conversion with the help of the following equation below. The SI base unit for amount of substance is the mole. To complete this calculation you have to know what substance you.
 Source: pinterest.com
Source: pinterest.com
Of Moles textMolar Mass Or. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. A common request on this site is to convert grams to moles. Solution Look for the atomic masses of hydrogen sulfur and oxygen. How To Convert Moles To Grams.
 Source: pinterest.com
Source: pinterest.com
Molecular weight of HgO or grams. Substitute 6 in 6 10 23. You can view more details on each measurement unit. To complete this calculation you have to know what substance you. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
 Source: pinterest.com
Source: pinterest.com
A common request on this site is to convert grams to moles. You can view more details on each measurement unit. Apr 15 2020 To transform moles into grams decide the variety of moles preset and the molar mass of the compound. Similarly we evaluate the moles using the compounds mass and considering its. A compounds moles are converted into mass in grams if its molar mass is known.
 Source: pinterest.com
Source: pinterest.com
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. This dimensional analysis video tuto. The answer is 00092705727916105. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. Type in your own numbers in the form to convert the units.
 Source: pinterest.com
Source: pinterest.com
To convert grams to moles. Convert another chemical substance Convert moles to grams Quick conversion chart of moles Br to grams 1 moles Br to grams 79904 grams 2 moles Br to grams 159808 grams 3 moles Br to grams 239712 grams 4 moles Br to grams 319616 grams 5 moles Br to grams 39952 grams. A common request on this site is to convert grams to moles. Convert mole to gram-force The reason is that the molar mass of the substance affects the conversion. This compound is also known as Mercury II Oxide.
 Source: pinterest.com
Source: pinterest.com
A compounds moles are converted into mass in grams if its molar mass is known. The SI base unit for amount of substance is the mole. 7 moles in to grams 803726 gramsBased on that information to convert 1009 moles of curium to grams we multiply 1009 moles of curium by 247. The SI base unit for amount of substance is the mole. Note that rounding errors may occur so always check the results.
 Source: pinterest.com
Source: pinterest.com
Type in your own numbers in the form to convert the units. Apr 15 2020 To transform moles into grams decide the variety of moles preset and the molar mass of the compound. The SI base unit for amount of substance is the mole. Once you have both the molecular mass and physical weight of the compound you can use a simple formula to calculate the number of moles present in the sample. In case you have x grams you devide x by grams per mole to get moles.
 Source: pinterest.com
Source: pinterest.com
You can view more details on each measurement unit. This compound is also known as Mercury II Oxide. The answer is 00092705727916105. Take the number of moles and multiply them by Avogadros number. Moles To Grams Formula.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to convert moles of a compound to grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.