Your How to convert mole percent to grams images are available in this site. How to convert mole percent to grams are a topic that is being searched for and liked by netizens now. You can Download the How to convert mole percent to grams files here. Get all free photos.
If you’re searching for how to convert mole percent to grams pictures information related to the how to convert mole percent to grams interest, you have come to the ideal site. Our site frequently provides you with suggestions for seeking the maximum quality video and picture content, please kindly hunt and find more informative video content and images that fit your interests.
How To Convert Mole Percent To Grams. Now we have to perform moles to grams calculation. 1 mole is equal to 1 moles Cholesterol or 38665354 grams. More commonly written for this application as. Interchanging Between Percent Concentration and Molarity.
 Hubert Hudson Fold Frog Convert Moles To Grams Calculator Uctsc Org From uctsc.org
Hubert Hudson Fold Frog Convert Moles To Grams Calculator Uctsc Org From uctsc.org
By calculating we get the molecular weight of CO 2801 gmol. More commonly written for this application as. Moles O 212 g O x 1 mol O 1600 g O 133 mol O The numbers of moles of each element are in the same ratio as the number of atoms Sn and O in cassiterite. Hence one mole of H 2 SO 4 weights 106076 grams. Now click the button Solve to get the conversion value. Since you need to find for 360 mol of H 2 SO 4 360 mol x 106076 gmol 38187 g of H2SO4.
You can also verify the results by fetching the.
02 x 5844 11688 grams. 250 moles x 122550 gmole 306375 grams The answer should be rounded off to three significant figures resulting in 306 g. The sum of the mole percentages for each component in a solution is equal to 100. X 850 gL. What you can do is calculate how many grams is n of a given number of. How do you find Percent change from molarity.
 Source: khanacademy.org
Source: khanacademy.org
X 850 gL. M 3899 107868. Mole a χa 100. Interchanging Between Percent Concentration and Molarity. They are incompatible units of conversion.
 Source: inchcalculator.com
Source: inchcalculator.com
W eight percent mass of the solute mass of the solution 100 W e i g h t p e r c e n t m a s s o f t h e s o l u t e m a s s o f t h e s o l u t i o n 100. X 85 gGMW x 10. Moles are a standard unit of measurement in chemistry that take into account the different elements in a chemical compound. W eight percent mass of the solute mass of the solution 100 W e i g h t p e r c e n t m a s s o f t h e s o l u t e m a s s o f t h e s o l u t i o n 100. Interchanging Between Percent Concentration and Molarity.
 Source: slidetodoc.com
Source: slidetodoc.com
Mole a χa 100. Interchanging Between Percent Concentration and Molarity. By calculating we get the molecular weight of CO 2801 gmol. 0700 mole x 340146 gramsmole 238 grams the answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures. Moles O 212 g O x 1 mol O 1600 g O 133 mol O The numbers of moles of each element are in the same ratio as the number of atoms Sn and O in cassiterite.
 Source: youtube.com
Source: youtube.com
Divide the mass of the dissolved compound by the mass of the solution and multiply the result by 100 to calculate percentage. Moles O 212 g O x 1 mol O 1600 g O 133 mol O The numbers of moles of each element are in the same ratio as the number of atoms Sn and O in cassiterite. More commonly written for this application as. Percentages are ratios literally ratios out of a hundred. When we solve the equation above for grams we get the percent to grams formula as follows.
 Source: youtube.com
Source: youtube.com
X 85 gGMW x 10. More commonly written for this application as. X 85 gGMW x 10. Hence one mole of H 2 SO 4 weights 106076 grams. For a solution containing two components solute and solvent mole solute mole solvent 100.
 Source: uctsc.org
Source: uctsc.org
Answer 1 of 3. Since we need to find. M 3899 107868. 00891 x 100 891g That means that I need to use 891g DPTAP and 9109g. 02 x 5844 11688 grams.
 Source: youtube.com
Source: youtube.com
You can also verify the results by fetching the. So if m is the mass of Solute and M is the mass of solvent then Weight percent formula will be. The sum of the mole percentages for each component in a solution is equal to 100. X 085 x 1000 100. 0700 mole x 340146 gramsmole 238 grams the answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures.
 Source: uctsc.org
Source: uctsc.org
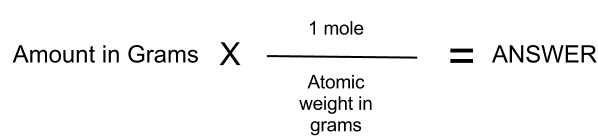
00891 x 100 891g That means that I need to use 891g DPTAP and 9109g. Grams Moles x Molar Mass. Use this page to learn. Note how the mole in the numerator and the mole in the denominator cancel. Molar Mass 107868.
 Source: youtube.com
Source: youtube.com
How do you find Percent change from molarity. Grams Moles x Molar Mass. Molarity g of soluteGMW of solute x 1 liter. So if m is the mass of Solute and M is the mass of solvent then Weight percent formula will be. 250 moles x 122550 gmole 306375 grams The answer should be rounded off to three significant figures resulting in 306 g.
 Source: uctsc.org
Source: uctsc.org
You can also verify the results by fetching the. The sum of the mole percentages for each component in a solution is equal to 100. M 420577332 g. Percentages are ratios literally ratios out of a hundred. 10mol percent x 64647 gmol 7252821 g mol 00891.
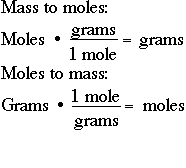 Source: uctsc.org
Source: uctsc.org
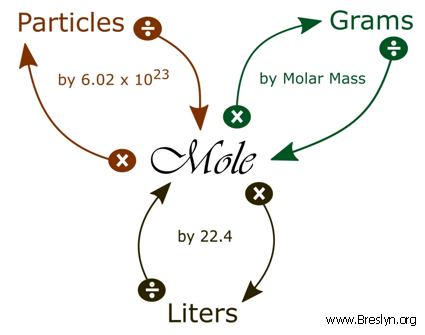
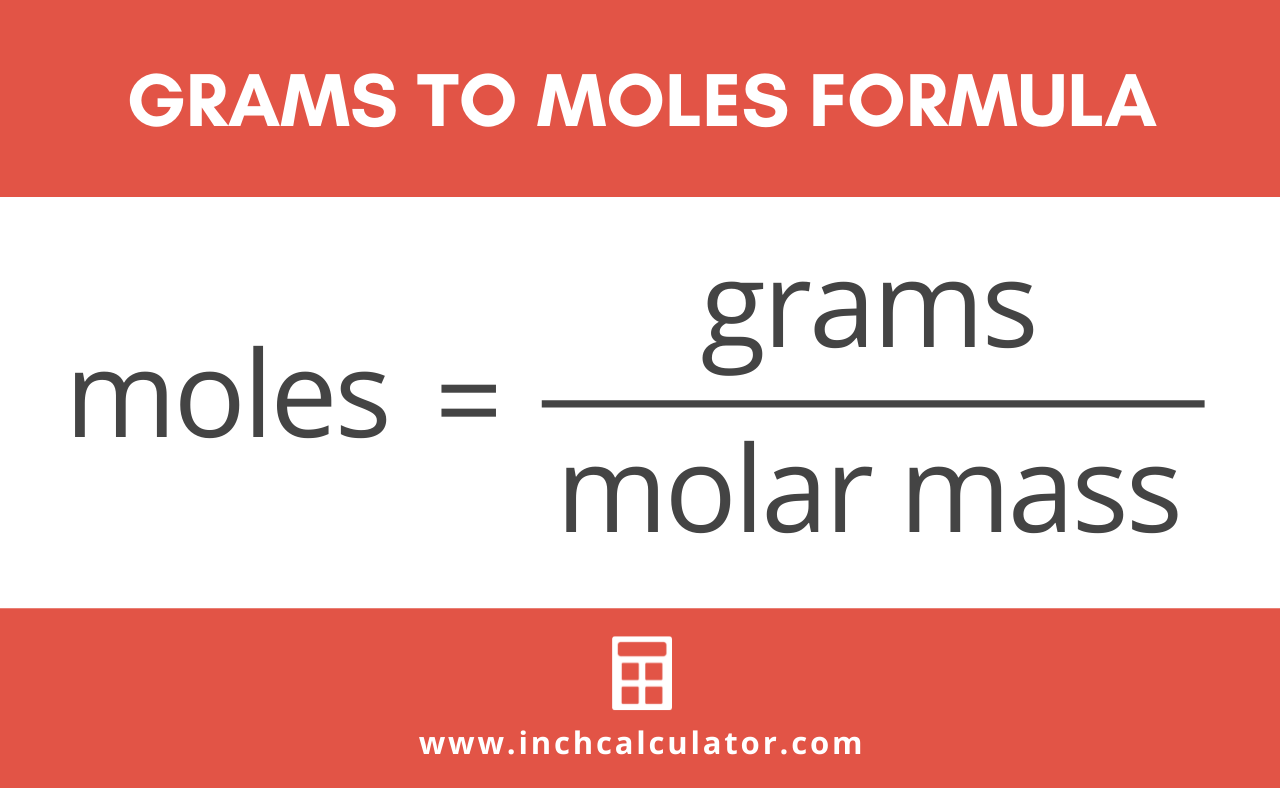
Moles O 212 g O x 1 mol O 1600 g O 133 mol O The numbers of moles of each element are in the same ratio as the number of atoms Sn and O in cassiterite. Divide the mass of the dissolved compound by the mass of the solution and multiply the result by 100 to calculate percentage. For a solution containing two components solute and solvent mole solute mole solvent 100. Percentages are ratios literally ratios out of a hundred. Moles to Grams Conversion FormulaIn order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass.
 Source: uctsc.org
Source: uctsc.org
For a solution containing two components solute and solvent mole solute mole solvent 100. The sum of the mole percentages for each component in a solution is equal to 100. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Since you need to find for 360 mol of H 2 SO 4 360 mol x 106076 gmol 38187 g of H2SO4. 1 mole is equal to 1 moles Cholesterol or 38665354 grams.
 Source: study.com
Source: study.com
To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. For calculations tap Molar Mass Calculator By using moles to grams formula. X 085 x 1000 100. Where is the molar mass of the substance.
 Source: uctsc.org
Source: uctsc.org
M 3899 107868. Now we have to perform moles to grams calculation. Where is the molar mass of the substance. So if m is the mass of Solute and M is the mass of solvent then Weight percent formula will be. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams.
 Source: researchgate.net
Source: researchgate.net
By calculating we get the molecular weight of CO 2801 gmol. Converting Percentages Into Grams Divide the percentage by 100 or equivalently move the decimal place two spots to the left to do this. Enter the moles and formula weight in the input field. Hence one mole of H 2 SO 4 weights 106076 grams. Moles to Grams Conversion FormulaIn order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to convert mole percent to grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.