Your How to convert molar solubility to grams per liter images are available in this site. How to convert molar solubility to grams per liter are a topic that is being searched for and liked by netizens today. You can Get the How to convert molar solubility to grams per liter files here. Find and Download all royalty-free photos.
If you’re looking for how to convert molar solubility to grams per liter pictures information connected with to the how to convert molar solubility to grams per liter topic, you have visit the ideal blog. Our site always provides you with hints for seeking the highest quality video and image content, please kindly surf and locate more informative video articles and images that match your interests.
How To Convert Molar Solubility To Grams Per Liter. So the maximum amount of calcium carbonate that is capable of dissolving in 1 liter of water at 25C is 67 10 -3 grams. In this case we calculate the solubility product by taking the solids solubility expressed in units of moles per liter molL known as its molar solubility. 297 x 10 10 grams 100mL x 1010 297 x 10 9 grams 1000mL 297 x 10 9 grams L divided by 9077 gmol 327 x 10 11 molL. 1 multiply the g100mL value by 1010.

Note that in the case above. Therefore that of F is 42 10 4 M that is twice the concentration of Ca2. Density of low viscous liquids can be measured by exact weighing of a well-defined volume of the liquid. Calculate the solubility in grams per liter. F molarity molliter solution G mole fraction mol solute mol total H gram of soluteliter solution Density is not needed for conversons between wt mole fraction and molality or between molarity and grams of solute per liter solution. 297 x 10 10 grams 100mL x 1010 297 x 10 9 grams 1000mL 297 x 10 9 grams L divided by 9077 gmol 327 x 10 11 molL.
I know I should divide g L 1 by the molar mass of the substance but I dont seem to find the specific answer on Google.
Gram per litre to Density of air at sea degree. We use molar mass which is grams per mole. GL to molL Conversion. Since there is a 11 molar ratio between it and silver carbonate the value for s is also the molar solubility of silver carbonate. You can view more similar questions or ask a new question. View the full answer.
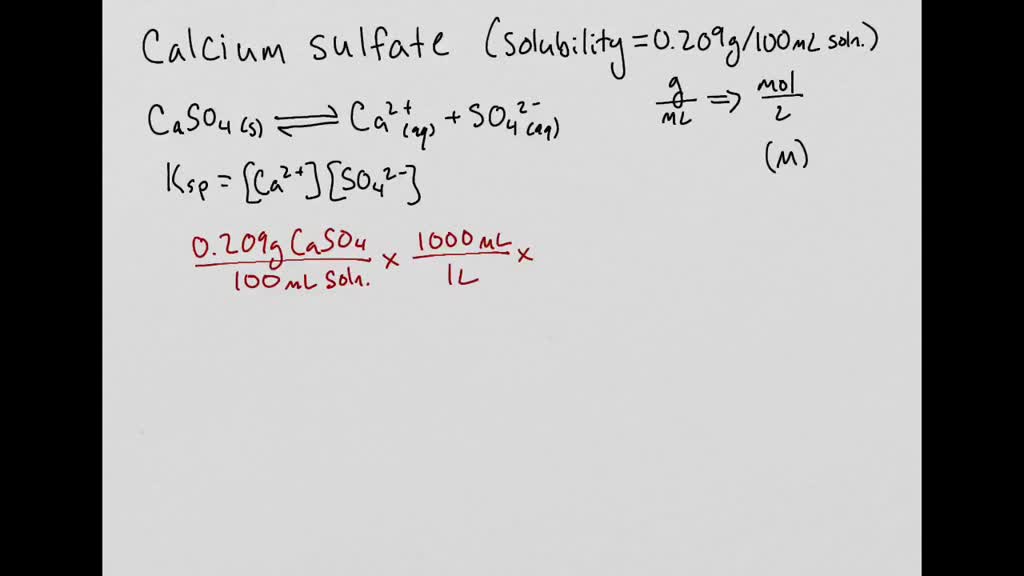 Source: numerade.com
Source: numerade.com
Solubility is normally expressed in gL of saturated solution. Gram per litre to Density of air at sea degree. So the maximum amount of calcium carbonate that is capable of dissolving in 1 liter of water at 25C is 67 10 -3 grams. This converts it to grams per 1000 mL or better yet grams per liter. However solubility can also be expressed as the moles per liter.
 Source: slideplayer.com
Source: slideplayer.com
Gram per litre to Density of air at sea degree. We can use the molar mass to convert from molar solubility to solubility. I know I should divide g L 1 by the molar mass of the substance but I dont seem to find the specific answer on Google. In other words the molar solubility of a given compound represents the highest molarity solution that is possible for that compound. Step 1- Change solubility in gramsL to molesL using Molar Mass.
 Source: youtube.com
Source: youtube.com
If I have 10 5 g L 1 C u X 2 solution do I have 157 10 7 m o l L 1. The concentration of Ca 2 in a saturated solution of CaF 2 is 21 10 4 M. Note that in the case above. So just to be sure. I m not sure how to convert M to gL MOLAR MASS OF COLLIGATIVE PROPERTIES.
 Source: slideplayer.com
Source: slideplayer.com
In this case we calculate the solubility product by taking the solids solubility expressed in units of moles per liter molL known as its molar solubility. Gram per litre to Density of air at sea degree. However solubility can also be expressed as the moles per liter. I know I should divide g L 1 by the molar mass of the substance but I dont seem to find the specific answer on Google. 297 x 10 10 grams 100mL x 1010 297 x 10 9 grams 1000mL 297 x 10 9 grams L divided by 9077 gmol 327 x 10 11 molL.
 Source: pinterest.com
Source: pinterest.com
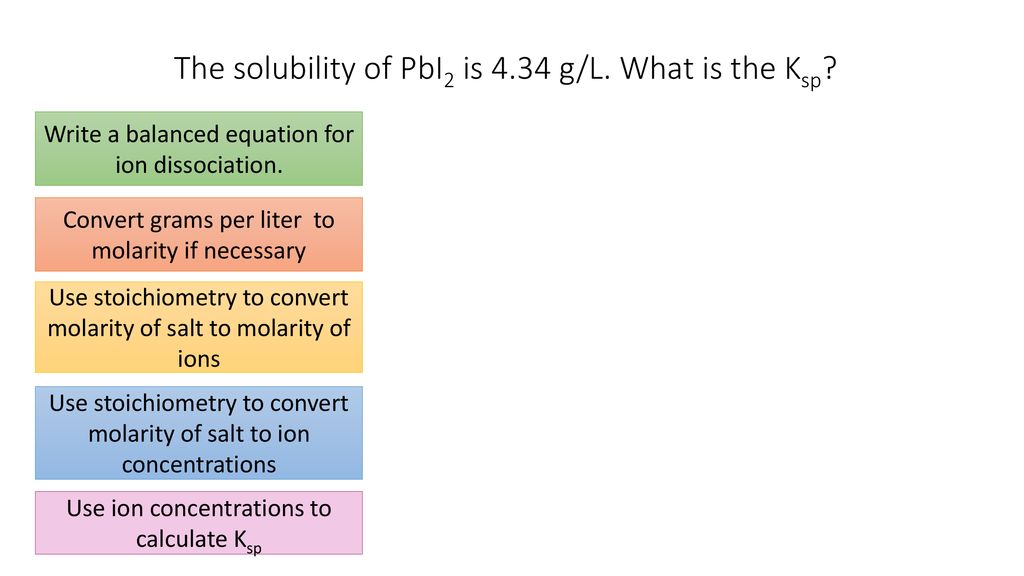
How to ger concentration molL of a solution from the mass grams of salt dissolved in water. Adding to that how do you convert solubility to grams per liter. View the full answer. I know I should divide g L 1 by the molar mass of the substance but I dont seem to find the specific answer on Google. So the maximum amount of calcium carbonate that is capable of dissolving in 1 liter of water at 25C is 67 10 -3 grams.
 Source: youtube.com
Source: youtube.com
1 multiply the g100mL value by 1010. View the full answer. You can convert between wv mv or weight ratio percentage concentration and molarity mol L-1 using the molar mass of the solute. I think Im missing something. Here is how to convert a g100mL value to molar solubility.
 Source: youtube.com
Source: youtube.com
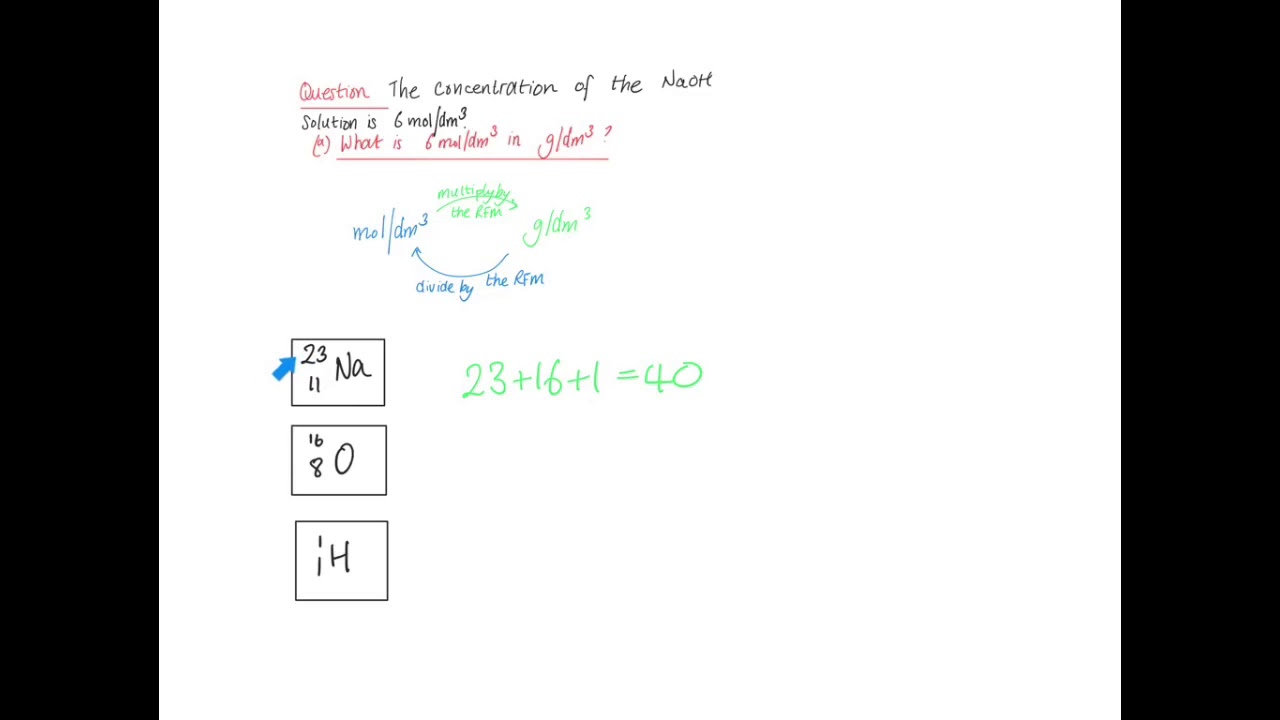
Density of low viscous liquids can be measured by exact weighing of a well-defined volume of the liquid. Here is how to convert a g100mL value to molar solubility. Moles to Grams Conversion FormulaIn order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. In this case we calculate the solubility product by taking the solids solubility expressed in units of moles per liter molL known as its molar solubility. What do we multiply moleslitre by to get gramslitre.
 Source: youtube.com
Source: youtube.com
This converts it to grams per 1000 mL or better yet grams per liter. This converts it to grams per 1000 mL or better yet grams per liter. However solubility can also be expressed as the moles per liter. Step 1- Change solubility in gramsL to molesL using Molar Mass. Where is the molar mass of the substance.

In the same way how do you calculate grams per liter. We can use the molar mass to convert from molar solubility to solubility. Moles per liter to grams per liter calculator. Might 05 2015 2 Answers2. Now we are already in litres so how to convert moles to grams.
 Source: slideplayer.com
Source: slideplayer.com
1 multiply the g100mL value by 1010. In other words the molar solubility of a given compound represents the highest molarity solution that is possible for that compound. Molar solubility is the number of moles of solute in one liter of saturated solution. Note that in the case above. Might 05 2015 2 Answers2.
 Source: youtube.com
Source: youtube.com
Molar Mass of Regularly Calculated Chemical substances. The concentration of Ca 2 in a saturated solution of CaF 2 is 21 10 4 M. Ksp to molar solubility or gL. Here is how to convert a g100mL value to molar solubility. You can convert between wv mv or weight ratio percentage concentration and molarity mol L-1 using the molar mass of the solute.
 Source: khanacademy.org
Source: khanacademy.org
Molar solubility is the number of moles of solute in one liter of saturated solution. This converts it to grams per 1000 mL or better yet grams per liter. 3 Convert from gL to g100mL. The molar mass of MnF2 549 2190 929 gramsmole 660 grams 1 mole 0071044 moles _ _. I think Im missing something.
 Source: nagwa.com
Source: nagwa.com
We can use the molar mass to convert from molar solubility to solubility. I know I should divide g L 1 by the molar mass of the substance but I dont seem to find the specific answer on Google. 2 Convert molL to gramL. Use the molar mass of NicO3 3768 x 10 118701 gmol to convert from moles per liter to grams per liter. In other words the molar solubility of a given compound represents the highest molarity solution that is possible for that compound.
 Source: youtube.com
Source: youtube.com
Calculate the solubility in grams per liter. I m not sure how to convert M to gL MOLAR MASS OF COLLIGATIVE PROPERTIES. 3 Convert from gL to g100mL. An insoluble salt with formula MX3 has a solubility product constant written in terms of. 10 grams to liter 001 liter.
 Source: pinterest.com
Source: pinterest.com
The Ksp of calcium carbonate is 45 10 -9. How one can ger focus molL of an answer from the mass grams of salt dissolved in water. An insoluble salt with formula MX3 has a solubility product constant written in terms of. Dec 02 2016 Water has a molarity of 555 M. Solubility is normally expressed in gL of saturated solution.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to convert molar solubility to grams per liter by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






