Your How to convert molar mass into atomic mass images are ready in this website. How to convert molar mass into atomic mass are a topic that is being searched for and liked by netizens today. You can Get the How to convert molar mass into atomic mass files here. Download all free vectors.
If you’re looking for how to convert molar mass into atomic mass images information connected with to the how to convert molar mass into atomic mass keyword, you have come to the ideal site. Our site always provides you with hints for seeing the highest quality video and picture content, please kindly surf and locate more informative video articles and images that match your interests.
How To Convert Molar Mass Into Atomic Mass. How to convert mass to mole. To convert grams into atoms you have to convert them into moles first. Atomic mass units u are used to measure mass of molecules and atoms. Molar mass symbol M is a physical property characteristic of a given substance chemical element or chemical compound namely its mass per amount of substance.
 Molar Mass Definition Formula Mole Atomic Mass Chemistrygod From chemistrygod.com
Molar Mass Definition Formula Mole Atomic Mass Chemistrygod From chemistrygod.com
A mole is 6022 1023 of something and this number is referred to as Avogadros number. Moles mol x Molar Mass gmol 1 x 5844. This site explains how to find molar mass. Da or u where 1 dalton is defined as 1 12 of the mass of a single carbon-12 atom at rest. The slug is a unit of mass used in the imperial system mainly in the UK. 1007 x 2 2014 grams per mole.
Convert molar mass to grams - Conversion of Measurement Units.
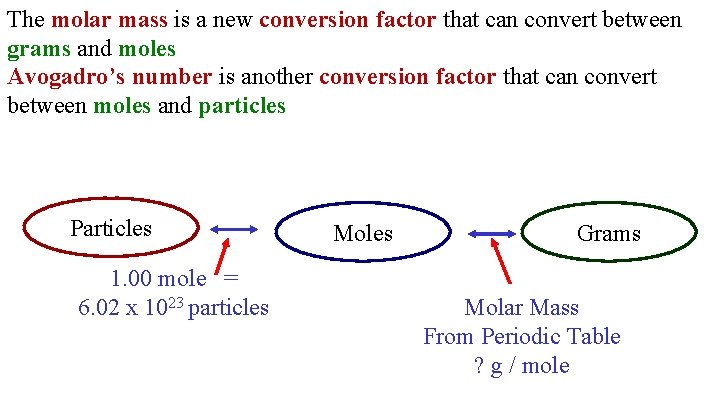
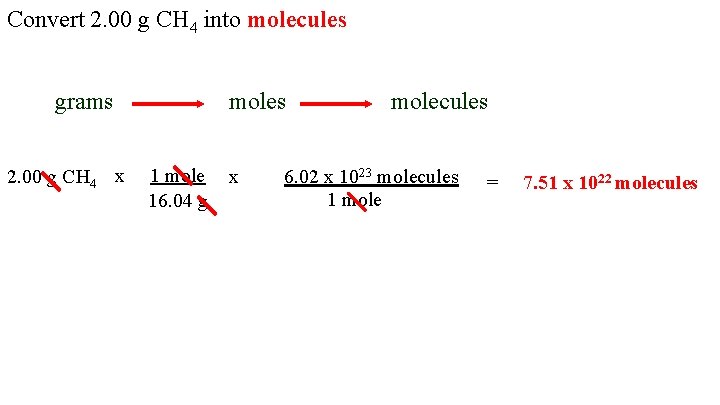
In other words it tells you the number of grams per mole of a compound. For example the atomic mass of the element neon listed on the periodic table on the inside cover of this book is 201797 giving a molar mass of 201797 gmol. The number of grams in the molar mass of an element is the same as the atomic mass. Thus the derived unit for molar mass is kgmol. To convert from moles to atoms multiply the molar amount by Avogadros number. The mass of 1 mol of hydrogen atoms is 10079 g.
 Source: slidetodoc.com
Source: slidetodoc.com
For NaCl the molar mass is 5844 gmol. 1007 x 2 2014 grams per mole. The molar mass of a compound tells you the mass of 1 mole of that substance. The atomic mass m a or m is the mass of an atomAlthough the SI unit of mass is kilogram symbol. The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in.
 Source: pinterest.com
Source: pinterest.com
The average atomic mass is measured in the unified mass unit u. In chemistry the molar mass of a chemical compound is defined as the mass of 1 mole or 60221410 23 particles of the substance expressed in grams. The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in. The molar mass of a compound tells you the mass of 1 mole of that substance. Kg the atomic mass is often expressed in the non-SI unit dalton symbol.
 Source: youtube.com
Source: youtube.com
To convert from moles to atoms multiply the molar amount by Avogadros number. I have EDX energy dispersive X-ray analysis for a thin film the results are in ms mass percentage how can I convert mass percentage to Stack Exchange Network Stack Exchange network consists of 178 QA communities including Stack Overflow the largest most trusted online community for developers to learn share their knowledge and. 1007 x 2 2014 grams per mole. Atomic mass units u are used to measure mass of molecules and atoms. Finding molar mass starts with units of grams per mole gmol.
 Source: slidetodoc.com
Source: slidetodoc.com
Molar mass symbol M is a physical property characteristic of a given substance chemical element or chemical compound namely its mass per amount of substance. It is about 166 10²⁷ kilograms. It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu. How do you find atoms in a molecule. To convert from moles to atoms multiply the molar amount by Avogadros number.
 Source: youtube.com
Source: youtube.com
Hydrogen H its atomic mass is 1008. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. It is defined to be 112 of the mass of one atom of carbon-12. Atomic mass units u are used to measure mass of molecules and atoms. An atomic mass is unit less and defined as precisely 112 the mass of an atom of carbon-12 not in motion.

The units for molar mass are therefore gramsmole. Mass g No. Average Atomic Mass to Molar mass. How to convert molarity into grams per liter - Quora. 1 slug is defined as a mass that accelerates by 1 foot per second squared when one pound.
 Source: pinterest.com
Source: pinterest.com
Molar mass symbol M is a physical property characteristic of a given substance chemical element or chemical compound namely its mass per amount of substance. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. 1 slug is defined as a mass that accelerates by 1 foot per second squared when one pound. How do you convert moles to liters per molarity. MOLAR MASS of Hydrogen 1007 gmol.
 Source: youtube.com
Source: youtube.com
It is defined to be 112 of the mass of one atom of carbon-12. MOLAR MASS OF AN ELEMENT RELATIVE ATOMIC MASS MOLAR MASS CONSTANT1gmol for HYDROGEN MOLAR MASS of Hydrogen 10071gmol. The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in. 1 slug is defined as a mass that accelerates by 1 foot per second squared when one pound. It is defined to be 112 of the mass of one atom of carbon-12.
 Source: pinterest.com
Source: pinterest.com
Lets figure out what the difference between molar mass and atomic mass is and learn to use molar mass as a conversion factor and stop guessing on how to con. Molar mass symbol M is a physical property characteristic of a given substance chemical element or chemical compound namely its mass per amount of substance. Kg the atomic mass is often expressed in the non-SI unit dalton symbol. Da or unified atomic mass unit u when describing atomic masses and molecular masses. I have EDX energy dispersive X-ray analysis for a thin film the results are in ms mass percentage how can I convert mass percentage to Stack Exchange Network Stack Exchange network consists of 178 QA communities including Stack Overflow the largest most trusted online community for developers to learn share their knowledge and.
 Source: pinterest.com
Source: pinterest.com
An atomic mass is unit less and defined as precisely 112 the mass of an atom of carbon-12 not in motion. Get the molar mass and multiply it by the number of moles to get the atoms. An atomic mass is unit less and defined as precisely 112 the mass of an atom of carbon-12 not in motion. The place is the molar mass of the substance. Average Atomic Mass to Molar mass.
 Source: chemistrygod.com
Source: chemistrygod.com
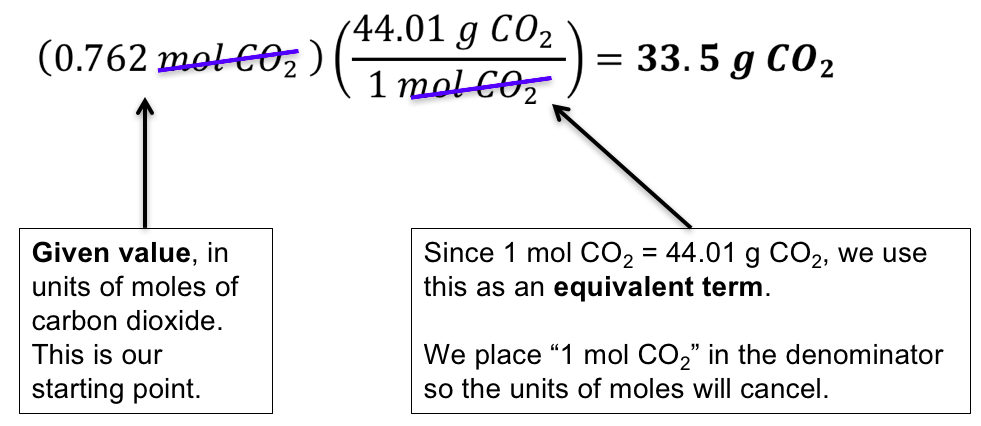
Molar mass symbol M is a physical property characteristic of a given substance chemical element or chemical compound namely its mass per amount of substance. If the average atomic mass m a or average molecular mass is known we can convert it into the molar mass M with the help of the molar mass constant M u. Convert molar mass to grams - Conversion of Measurement Units. Moles mol x Molar Mass gmol 1 x 5844. This site explains how to find molar mass.
 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
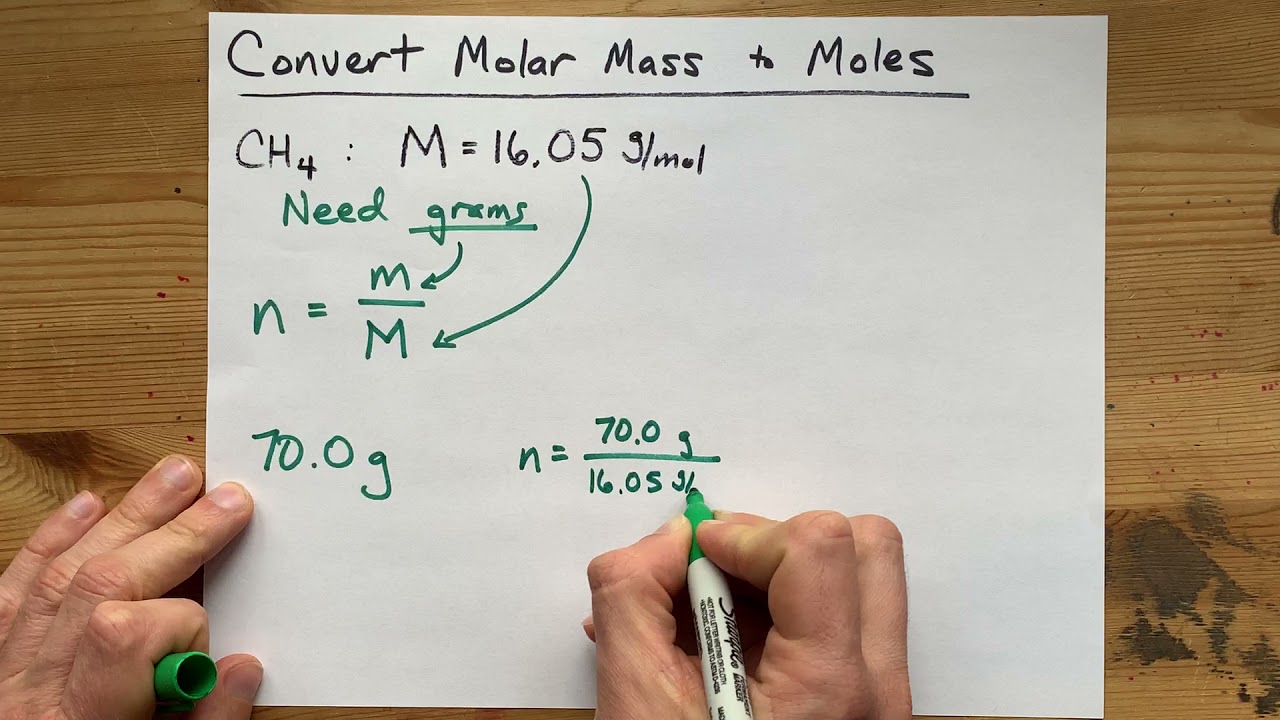
If the average atomic mass m a or average molecular mass is known we can convert it into the molar mass M with the help of the molar mass constant M u. Translating atomic plenty into molar plenty you may assemble conversion elements that convert between the mass of a component and the variety of moles of the factor. The average atomic mass is measured in the unified mass unit u. 1 u is 112 of a mass of an atom of carbon-12. It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu.
 Source: youtube.com
Source: youtube.com
In other words it tells you the number of grams per mole of a compound. Atomic mass units u are used to measure mass of molecules and atoms. But if youre converting from grams then the measure is gramsmole on the periodic table thus you take the amount of grams you have and divide it by the respective elements molar mass or atomic mass which varies depending on the element Hydrogen has an atomic weight of 1008 gmol vs Oxygen 15998 gmol due to their differing sizes caused by increasing the number of. Mass g No. A mole is 6022 1023 of something and this number is referred to as Avogadros number.
 Source: pinterest.com
Source: pinterest.com
An atomic mass is unit less and defined as precisely 112 the mass of an atom of carbon-12 not in motion. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. A mole is 6022 1023 of something and this number is referred to as Avogadros number. Translating atomic masses into molar masses you can construct conversion factors that convert between the mass of an element and the number of moles of the element. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal.
 Source: pinterest.com
Source: pinterest.com
Translating atomic masses into molar masses you can construct conversion factors that convert between the mass of an element and the number of moles of the element. Atomic mass units u are used to measure mass of molecules and atoms. 1 slug is defined as a mass that accelerates by 1 foot per second squared when one pound. The units for molar mass are therefore gramsmole. In chemistry the molar mass of a chemical compound is defined as the mass of 1 mole or 60221410 23 particles of the substance expressed in grams.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to convert molar mass into atomic mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






