Your How to convert mass to moles to particles images are ready. How to convert mass to moles to particles are a topic that is being searched for and liked by netizens now. You can Get the How to convert mass to moles to particles files here. Download all royalty-free photos and vectors.
If you’re searching for how to convert mass to moles to particles pictures information related to the how to convert mass to moles to particles interest, you have pay a visit to the ideal site. Our website frequently gives you hints for seeing the maximum quality video and image content, please kindly surf and locate more informative video content and images that fit your interests.
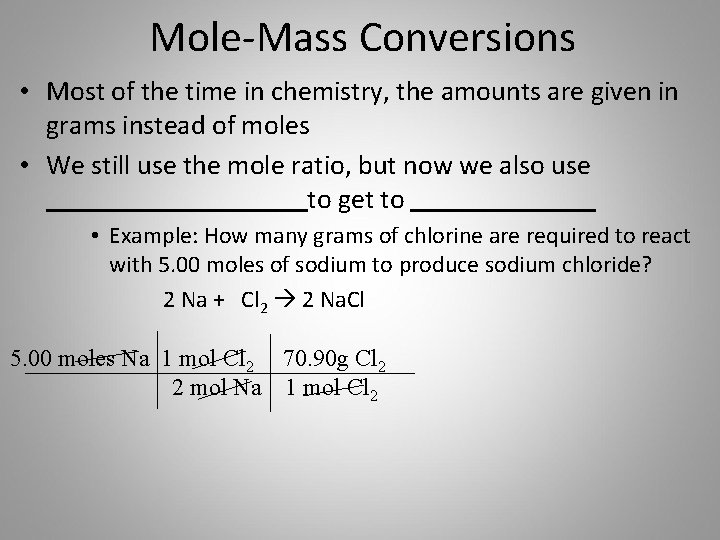
How To Convert Mass To Moles To Particles. Mass number of moles molar mass. Causey shows you step by step how to go from grams of a substance to moles of that substance to particles of that s. We can combine the two types of problems into one. Round to the nearest whole number.
 Mole Cube Chemistry Projects Chemistry Lessons Teaching Chemistry From pinterest.com
Mole Cube Chemistry Projects Chemistry Lessons Teaching Chemistry From pinterest.com
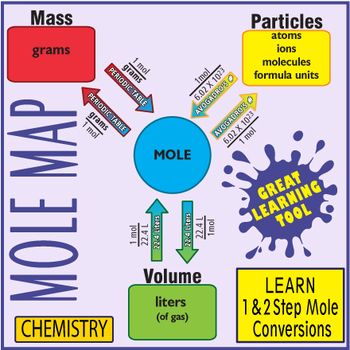
We can use the above equation to find the mass of a substance when we are given the number of moles of the substance. A significant feature of this mole conversion calculator is that it also determines the number of particles atoms molecules etc of the substance along with handling a large number of measurement units for mass ie. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. Now you have seen how to convert back and forth between moles and the mass of a substance in grams. The following diagram shows how to convert between mass mole and number of particles. Particles typically represents atoms molecules covalent and formula units fu ionic compounds Going from particles to moles you divide by the 6021023 particlesmol Going from moles to particles you multiply by 6021023 particlesmol.
Moles to Mass Calculation.
Now you have seen how to convert back and forth between moles and the mass of a substance in grams. Molar mass or molar weight is the mass that one mole of a substance has and they are defined in grams per mole. 1 mole 60221023 6022 10 23 atoms molecules protons etcTo convert from moles to atoms multiply the molar amount by Avogadros number. Now you have seen how to convert back and forth between moles and the mass of a substance in grams. Quick Reference Guide For Calculating And Converting Moles. Calculate the mass of a 2 moles and b 025 moles of iron.
 Source: pinterest.com
Source: pinterest.com
6021023 Now one mole 6021023 particles So multiply the of moles you have by Avogadros number and you have your answer. If 1 mole of atoms is 602 x 1023 atoms then 2 moles of atoms would be equal to 12 x 1024 atoms. Quick Reference Guide For Calculating And Converting Moles. 734 X 1044 fu. Add them and you have the molar mass of.
 Source: pinterest.com
Source: pinterest.com
Particles atoms Formula units molecules Moles Mass grams Divide by 602 X 1023 Multiply by 602 X 1023 Multiply by molar mass from periodic table Divide by molar mass from periodic table 35 dozen roses. Molar mass or molar weight is the mass that one mole of a substance has and they are defined in grams per mole. In other words 602 x 10 23 particles 1 mole. Particles typically represents atoms molecules covalent and formula units fu ionic compounds Going from particles to moles you divide by the 6021023 particlesmol Going from moles to particles you multiply by 6021023 particlesmol. Convert the mass of each element to moles using the molar mass from the periodic table.
 Source: pinterest.com
Source: pinterest.com
6021023 Now one mole 6021023 particles So multiply the of moles you have by Avogadros number and you have your answer. Quick Reference Guide For Calculating And Converting Moles. Particles atoms Formula units molecules Moles Mass grams Divide by 602 X 1023 Multiply by 602 X 1023 Multiply by molar mass from periodic table Divide by molar mass from periodic table 35 dozen roses. Convert the mass of each element to moles using the molar mass from the periodic table. How to convert mass to mole In chemistry the molar mass of a chemical compound is defined as the mass of 1 mole or 60221410 23 particles of the substance expressed in grams.
 Source: pinterest.com
Source: pinterest.com
Answer below First you need to know Avogadros number which is directly associated with molarity. Moles Mass and Particles Worksheet Answer Key 1 13 x 1023 formula units 2 191 x 1024 formula units 3 41 x 102 g 4 21 x 102 g 5 12 x 102 g 6 392 x 1023 formula units 7 31 x 102 g 8 107 x 1024 formula units 9 17 x 1022 formula units 10 43 x 102 g 11 782 x 1023 molecules 12 200 g 13 171 g 14 133 x 1023 formula units. Quick Reference Guide For Calculating And Converting Moles. Where mass is in grams and the molar mass is in grams per mole. In order to convert from mass to number of particles or vice-versa a conversion to moles is required.
 Source: pinterest.com
Source: pinterest.com
EXAMPLES OF MOLE - PARTICLE CONVERSIONS Particles to Moles Example 5 How many moles are in 201 X 1052 atoms of silver. The following diagram shows how to convert between mass mole and number of particles. To convert moles to particles atoms or molecules. Particles atoms Formula units molecules Moles Mass grams Divide by 602 X 1023 Multiply by 602 X 1023 Multiply by molar mass from periodic table Divide by molar mass from periodic table 35 dozen roses. Quick Reference Guide For Calculating And Converting Moles.
 Source: pinterest.com
Source: pinterest.com
In other words 602 x 10 23 particles 1 mole. EXAMPLES OF MOLE - PARTICLE CONVERSIONS Particles to Moles Example 5 How many moles are in 201 X 1052 atoms of silver. 372 AICI313333 mol 0279 mol AICI3 Is this correct. The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in daltons. 201 X 1052 atom Ag 1 mol Ag 602 X 1023 atom Ag 334 X 1028 mol Ag Example 6 How many moles are in 734 X 1044 formula units of calcium phosphate.
 Source: pinterest.com
Source: pinterest.com
Molar mass or molar weight is the mass that one mole of a substance has and they are defined in grams per mole. G lbs stone oz ton etc as. We can combine the two types of problems into one. Answer below First you need to know Avogadros number which is directly associated with molarity. Round to the nearest whole number.
 Source: pinterest.com
Source: pinterest.com
A significant feature of this mole conversion calculator is that it also determines the number of particles atoms molecules etc of the substance along with handling a large number of measurement units for mass ie. Therefore- number of particles in mol of NaCl 602E23 to find mass of a molecule in NaCl take the molar mass of Na and Cl. G lbs stone oz ton etc as. Molar mass â 1 mol. Divide each mole value by the smallest number of moles calculated.
 Source: pinterest.com
Source: pinterest.com
This is the mole ratio of the elements and is. A significant feature of this mole conversion calculator is that it also determines the number of particles atoms molecules etc of the substance along with handling a large number of measurement units for mass ie. Particles typically represents atoms molecules covalent and formula units fu ionic compounds Going from particles to moles you divide by the 6021023 particlesmol Going from moles to particles you multiply by 6021023 particlesmol. 201 X 1052 atom Ag 1 mol Ag 602 X 1023 atom Ag 334 X 1028 mol Ag Example 6 How many moles are in 734 X 1044 formula units of calcium phosphate. Quick Reference Guide For Calculating And Converting Moles.
 Source: pinterest.com
Source: pinterest.com
The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in daltons. Convert the mass of each element to moles using the molar mass from the periodic table. Mass and number of particles are both related to grams. We can combine the two types of problems into one. 201 X 1052 atom Ag 1 mol Ag 602 X 1023 atom Ag 334 X 1028 mol Ag Example 6 How many moles are in 734 X 1044 formula units of calcium phosphate.
 Source: pinterest.com
Source: pinterest.com
Changing Grams to Moles to Particles. This is the mole ratio of the elements and is. EXAMPLES OF MOLE - PARTICLE CONVERSIONS Particles to Moles Example 5 How many moles are in 201 X 1052 atoms of silver. To convert particles atoms or molecules to moles. The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in daltons.
 Source: pinterest.com
Source: pinterest.com
You cannot convert molar mass directly to moles. Mass number of moles molar mass. 734 X 1044 fu. Particles atoms Formula units molecules Moles Mass grams Divide by 602 X 1023 Multiply by 602 X 1023 Multiply by molar mass from periodic table Divide by molar mass from periodic table 35 dozen roses. We can combine the two types of problems into one.
 Source: pinterest.com
Source: pinterest.com
We can combine the two types of problems into one. Mass number of moles molar mass. Avogadros number is a very important relationship to remember. 372 AICI313333 mol 0279 mol AICI3 Is this correct. How to convert mass to mole In chemistry the molar mass of a chemical compound is defined as the mass of 1 mole or 60221410 23 particles of the substance expressed in grams.
 Source: pinterest.com
Source: pinterest.com
Molar mass or molar weight is the mass that one mole of a substance has and they are defined in grams per mole. To convert moles to particles atoms or molecules. 1 mole 60221023 6022 10 23 atoms molecules protons etcTo convert from moles to atoms multiply the molar amount by Avogadros number. Ca3PO42 1 mol Ca3PO42 602 X 1023 fu. Changing Grams to Moles to Particles.
 Source: pinterest.com
Source: pinterest.com
In other words 602 x 10 23 particles 1 mole. Convert the mass of each element to moles using the molar mass from the periodic table. The following diagram shows how to convert between mass mole and number of particles. Divide each mole value by the smallest number of moles calculated. Mass gformula unit AICI3 unknown The ratio of AI3 Ions to CI- Ions is 13 Molar Mass AICI3 1 x 2698 gmol AI 3 x 3545 gmol CI 13333 gmol AICI3 molar mass 13333 gmol AICI3 Divide the given mass of aluminum chloride by its molar mass.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to convert mass to moles to particles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.