Your How to convert grams to moles using avogadros number images are ready. How to convert grams to moles using avogadros number are a topic that is being searched for and liked by netizens now. You can Get the How to convert grams to moles using avogadros number files here. Download all royalty-free photos and vectors.
If you’re looking for how to convert grams to moles using avogadros number pictures information related to the how to convert grams to moles using avogadros number topic, you have visit the right blog. Our website always gives you hints for seeing the highest quality video and picture content, please kindly hunt and find more enlightening video articles and images that fit your interests.
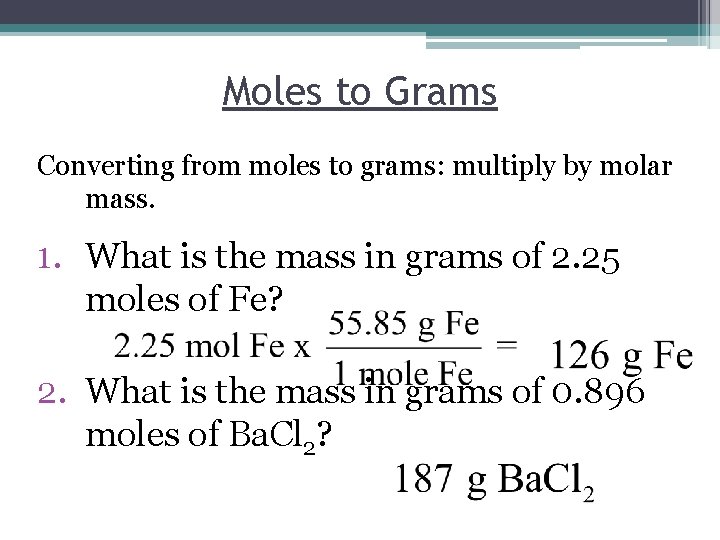
How To Convert Grams To Moles Using Avogadros Number. So a mole of carbon C would have a mass of 120107 g and contain 6022 X. Answer 1 of 2. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. To convert grams to moles.
 Beginners Chemistry Visual Aid For Teaching Introductory Conversions Between Atoms Moles And Grams Teaching Chemistry Chemistry Lessons Chemistry Education From pinterest.com
Beginners Chemistry Visual Aid For Teaching Introductory Conversions Between Atoms Moles And Grams Teaching Chemistry Chemistry Lessons Chemistry Education From pinterest.com
You can convert the grams to molecules by following the steps below. So a mole of carbon C would have a mass of 120107 g and contain 6022 X. The following diagram shows how to convert between Mass Mole and Number of particles. There are two moles of hydrogen atoms per one mole of water. The Avogadros number full is equal to 602214076 10 23. Take the number of moles and multiply them by Avogadros number.
Avogadros number is a very important relationship to remember.
This video also shows you how to calculate t. There are exactly 31773 grams in the given quantity of copper that could also be calculated by using a free online moles to grams calculator. 1 mole 60221023 6022 10 23 atoms molecules protons etcTo convert from moles to atoms multiply the molar amount by Avogadros number. Finally multiply by Avogadros number. The following diagram shows how to convert between Mass Mole and Number of particles. Avogadros number is a very important relationship to remember.
 Source: pinterest.com
Source: pinterest.com
We have 500mL of water. Using Avogadros number as a conversion factor Now that you understand what moles represent and why its useful to scale to that unit lets explore the application of this new conversion factor. How do you convert grams to molecules. Start by using the subscripts in the chemical formula C6H14O to determine the number of moles of hydrogen in 0170 mol of C6H14O then set up a conversion to cancel moles of hydrogen and convert to atoms using Avogadros number. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal.
 Source: pinterest.com
Source: pinterest.com
Take the number of moles and multiply them by Avogadros number. This video also shows you how to calculate t. First of all find the number of moles by using mass and molar mass. The following diagram shows how to convert between Mass Mole and Number of particles. Then use the molar mass of water to convert this to moles.
 Source: pinterest.com
Source: pinterest.com
To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. Moreover this number is also known as the Avogadros constant and is the number of atoms that are found to be existing in exactly 12 grams of carbon-12. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. The unit read as either inverse mole or per mole reminds us that there are 602 10 23 objects per one mole. The Avogadros number full is equal to 602214076 10 23.
 Source: pinterest.com
Source: pinterest.com
Avogadros number can be used as a conversion factor between moles and atoms as shown here. Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. Take the number of moles and multiply them by Avogadros number. Then use the molar mass of water to convert this to moles. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula.
 Source: pinterest.com
Source: pinterest.com
In 1902 Ostwald proposed the term mole as another way to express Avogadros Number. To convert from atoms to moles divide the atom amount by Avogadros number or multiply by its reciprocal. Avogadros number is a very important relationship to remember. H 0170 mol C6H14O1 14 mol H. 1 mole 60221023 6022 10 23 atoms molecules protons etc.
 Source: pinterest.com
Source: pinterest.com
Grams x 602 x 1023 mass number of specific element in grams Wiki User. Furthermore Avogadros number refers to the number of particles that exist in one mole of any substance. N m M where. In 1902 Ostwald proposed the term mole as another way to express Avogadros Number. Avogadros number can be used as a conversion factor between moles and atoms as shown here.
 Source: pinterest.com
Source: pinterest.com
Avogadros number is a very important relationship to remember. Divide the initial mass of the substance by the compounds molar mass listed in the periodic table of the elements. Grams x 602 x 1023 mass number of specific element in grams Wiki User. So a mole of carbon C would have a mass of 120107 g and contain 6022 X. Then use the molar mass of water to convert this to moles.
 Source: pinterest.com
Source: pinterest.com
To convert from moles to atoms multiply the molar amount by Avogadros number. H 0170 mol C6H14O1 14 mol H. Use the density of water the molar mass of water and Avogadros number to calculate the number of molecules of water. Answer 1 of 2. Grams x 602 x 1023 mass number of specific element in grams Wiki User.
 Source: pinterest.com
Source: pinterest.com
We have 500mL of water. There are exactly 31773 grams in the given quantity of copper that could also be calculated by using a free online moles to grams calculator. In 1902 Ostwald proposed the term mole as another way to express Avogadros Number. After doing so multiply the moles with the Avogadros. Use the density to convert this to grams.
 Source: pinterest.com
Source: pinterest.com
Using Avogadros number as a conversion factor Now that you understand what moles represent and why its useful to scale to that unit lets explore the application of this new conversion factor. Start by using the subscripts in the chemical formula C6H14O to determine the number of moles of hydrogen in 0170 mol of C6H14O then set up a conversion to cancel moles of hydrogen and convert to atoms using Avogadros number. Grams x 602 x 1023 mass number of specific element in grams Wiki User. Furthermore Avogadros number refers to the number of particles that exist in one mole of any substance. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula.
 Source: pinterest.com
Source: pinterest.com
Moreover this number is also known as the Avogadros constant and is the number of atoms that are found to be existing in exactly 12 grams of carbon-12. This number is known as Avogadros number in honor of Amedeo Avogadro. Avogadros number is a very important relationship to remember. Use the density to convert this to grams. This video also shows you how to calculate t.
 Source: pinterest.com
Source: pinterest.com
Finally multiply by Avogadros number. You can convert the grams to molecules by following the steps below. This is often associated with atoms andor molecules but you can apply it to others too although it. How do you convert grams to molecules. To convert from moles to atoms multiply the molar amount by Avogadros number.
 Source: pinterest.com
Source: pinterest.com
The Avogadros number full is equal to 602214076 10 23. Take the number of moles and multiply them by Avogadros number. To convert from moles to atoms multiply the molar amount by Avogadros number. Grams x 602 x 1023 mass number of specific element in grams Wiki User. There are two moles of hydrogen atoms per one mole of water.
 Source: pinterest.com
Source: pinterest.com
Start by using the subscripts in the chemical formula C6H14O to determine the number of moles of hydrogen in 0170 mol of C6H14O then set up a conversion to cancel moles of hydrogen and convert to atoms using Avogadros number. If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadros number of 602214154 x 10 23 particles per mole. 1 mole 60221023 6022 10 23 atoms molecules protons etcTo convert from moles to atoms multiply the molar amount by Avogadros number. The number 6022 X 10 23 was given the name Avogadros Number by Jean Perrin in 1901. The unit read as either inverse mole or per mole reminds us that there are 602 10 23 objects per one mole.
 Source: pinterest.com
Source: pinterest.com
The following diagram shows how to convert between Mass Mole and Number of particles. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. Its the number of particles contained in one mole of substance. Avogadros number is a very important relationship to remember. This general chemistry video tutorial focuses on avogadros number and how its used to convert moles to atoms.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to convert grams to moles using avogadros number by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.