Your How to convert grams to moles of an unknown substance images are available. How to convert grams to moles of an unknown substance are a topic that is being searched for and liked by netizens today. You can Find and Download the How to convert grams to moles of an unknown substance files here. Get all royalty-free photos and vectors.
If you’re looking for how to convert grams to moles of an unknown substance images information linked to the how to convert grams to moles of an unknown substance interest, you have visit the ideal site. Our site always gives you suggestions for seeking the maximum quality video and picture content, please kindly search and find more enlightening video content and images that fit your interests.
How To Convert Grams To Moles Of An Unknown Substance. See an example of converting grams to moles. 2 moles butane one can find the molar amount of oxygen 605 moles that reacts with 540 grams of butane. First of all you should check there are how many grams available in the problem. In order to determine the number of moles of a given compound the first thing you need to do is find the molecular mass or molecular weight of the compound in question.
 Unit 2 Section C Mole To Gram Conversions Or How To Count From studylib.net
Unit 2 Section C Mole To Gram Conversions Or How To Count From studylib.net
Moles to Grams Conversion FormulaIn order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. If the answer is asked for in grams convert from moles to grams. Mole to mole 4. Molar mass has units of grams per mole gmol. Mass to mole 3. Where is the molar mass of the substance.
Where is the molar mass of the substance.
If you are given the mass of one substance convert from grams to moles. Compounds on a standard scale. This is equal to the number of grams in one mole of the substance. Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have. Where is the molar mass of the substance. Note that all formulas are case-sensitive.
 Source: studylib.net
Source: studylib.net
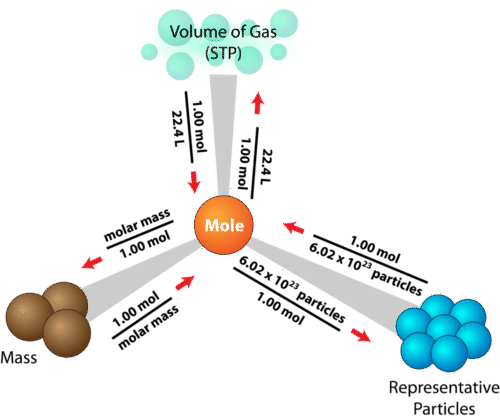
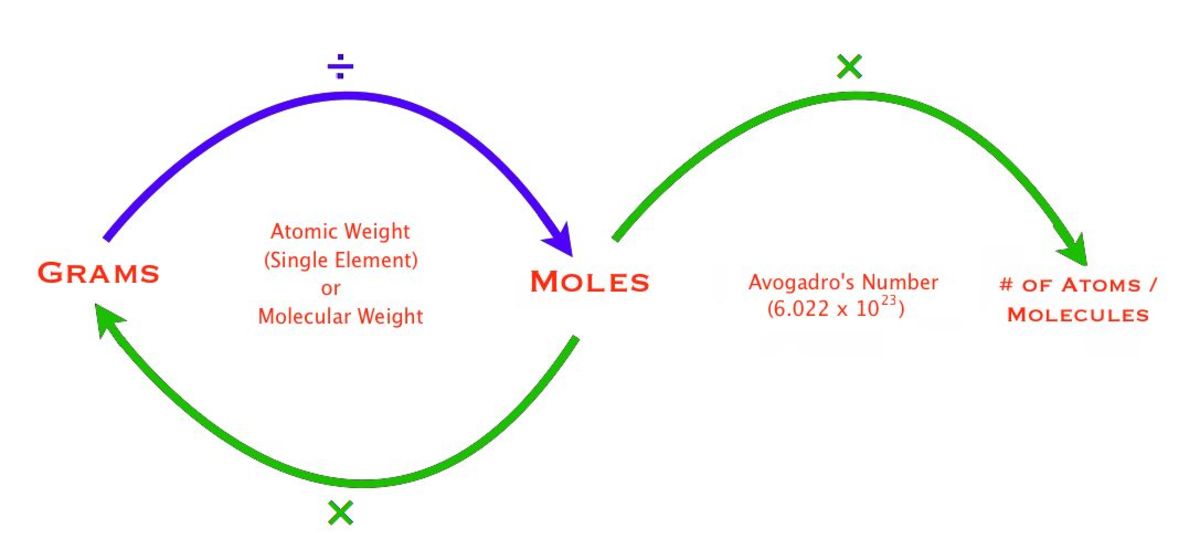
This will be important to know when converting from grams to moles to atoms. Moles Units and Moles Units and Conversion FactorsConversion Factors Presented byPresented by. Here are a few simple steps that can be used to convert the grams into moles. This is equal to the number of grams in one mole of the substance. Compounds on a standard scale.
 Source: youtube.com
Source: youtube.com
Convert from moles of the given substance to moles of the unknown substance using the appropriate mole ratio from the balanced chemical equation as the conversion factor. 20 molesliter 05L 1mol NaCl. 1 grams Kl mole using the molecular weight calculator and the molar mass of Kl. Mass to mole 3. Now divide the step one by step two.
 Source: youtube.com
Source: youtube.com
Divide the number of grams of the compound by its molecular mass. If you are given the mass of one substance convert from grams to moles. The relations among amount of substance in moles mass in. See an example of converting grams to moles. Now divide the step one by step two.
 Source: wikihow.com
Source: wikihow.com
Moles to Grams Conversion FormulaIn order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. Molar mass has units of grams per mole gmol. Application question 1 How many grams of NaCl are required to make 500mL of a 20M solution. Do a quick conversion. This will give you the molecular mass of the molecule.
 Source: khanacademy.org
Source: khanacademy.org
When we do this kind of conversion we call it stoic. When we do this kind of conversion we call it stoic. Here are a few simple steps that can be used to convert the grams into moles. Try another chemical formula or view the list of known compounds. Do a quick conversion.
 Source: wikihow.com
Source: wikihow.com
The relations among amount of substance in moles mass in. Try another chemical formula or view the list of known compounds. Converting grams to moles involves 2 steps. Do a quick conversion. Now you need to find the molar mas of the substance.
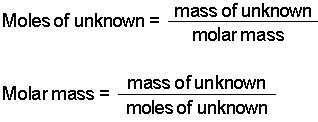 Source: chem.purdue.edu
Source: chem.purdue.edu
Find the molecular mass of the compound. 1 grams SOLuTe 0002851997853016 mole using the molecular weight calculator and the molar mass of SOLuTe. Try another chemical formula or view the list of known compounds. Avogadros principletells us that 1 mole is equal to 6022x1023 atoms of a pure substance. If the answer is asked for in grams convert from moles to grams.
 Source: slidetodoc.com
Source: slidetodoc.com
Compounds on a standard scale. N m M where. Convert to liters then multiply by density of substance. 1 grams SOLuTe 0002851997853016 mole using the molecular weight calculator and the molar mass of SOLuTe. Moles to Grams Conversion FormulaIn order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass.
 Source: wikihow.com
Source: wikihow.com
Determine amount of substance needed Calculate limiting reactants. Molar mass has units of grams per mole gmol. The molar mass M of a substance is the mass of one mole of entities atoms molecules or formula units of the substance. As the correct answer. In summary it was impossible to directly determine the mass of oxygen that could react with 540 grams of butane.
The answer will be the number of moles of the compound. Note how the mole in the numerator and the mole in the denominator cancel. CO2 - Converting. Application question 1 How many grams of NaCl are required to make 500mL of a 20M solution. Molar mass has units of grams per mole gmol.
 Source: discover.hubpages.com
Source: discover.hubpages.com
The answer will be the number of moles of the compound. Find the molecular mass of the compound. Convert to liters then multiply by density of substance. The molar mass M of a substance is the mass of one mole of entities atoms molecules or formula units of the substance. Now divide the step one by step two.
 Source: youtube.com
Source: youtube.com
Now divide the step one by step two. Molar mass equals mass of substance per 1 mole of substanceGrams x Molar Mass of Substance Moles of SubstanceTake the moles of the substance and multiply it by Avogadros Number the number. With the grams use the molesmassmolar mass to find moles What is the formula to convert grams to moles. Divide grams by the molar mass. We use a balanced chemical equation to accomplish changing from mol of one substance to mol of another.
 Source: youtube.com
Source: youtube.com
Convert from moles of the given substance to moles of the unknown substance using the appropriate mole ratio from the balanced chemical equation as the conversion factor. More commonly written for this application as. The answer will be the number of moles of the compound. As the correct answer. But by converting the butane mass to moles 0929 moles and using the molar ratio 13 moles oxygen.
 Source: slideplayer.com
Source: slideplayer.com
1 grams Kl mole using the molecular weight calculator and the molar mass of Kl. 1 grams Kl mole using the molecular weight calculator and the molar mass of Kl. Mass to mole 3. The molar mass M of a substance is the mass of one mole of entities atoms molecules or formula units of the substance. Convert from moles of the given substance to moles of the unknown substance using the appropriate mole ratio from the balanced chemical equation as the conversion factor.
 Source: khanacademy.org
Source: khanacademy.org
Mole to mole 4. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. When we do this kind of conversion we call it stoic. This will be important to know when converting from grams to moles to atoms. First of all you should check there are how many grams available in the problem.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to convert grams to moles of an unknown substance by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





