Your How to convert grams to molecules in stoichiometry images are ready. How to convert grams to molecules in stoichiometry are a topic that is being searched for and liked by netizens now. You can Get the How to convert grams to molecules in stoichiometry files here. Download all free images.
If you’re searching for how to convert grams to molecules in stoichiometry images information related to the how to convert grams to molecules in stoichiometry keyword, you have visit the right blog. Our site frequently gives you suggestions for seeking the maximum quality video and image content, please kindly search and find more enlightening video content and images that fit your interests.
How To Convert Grams To Molecules In Stoichiometry. Limiting Reagent Examples Al — 100 g 26982 gmol 037062 mol O 3 — 190 g 47997 gmol 039586 mol 3 Determine limiting reagent. First the molar mass allows you to change mass into mol. Converting moles and mass. We can use this information to convert grams to molecules or liters molecules to grams or liters or liters to grams or molecules.
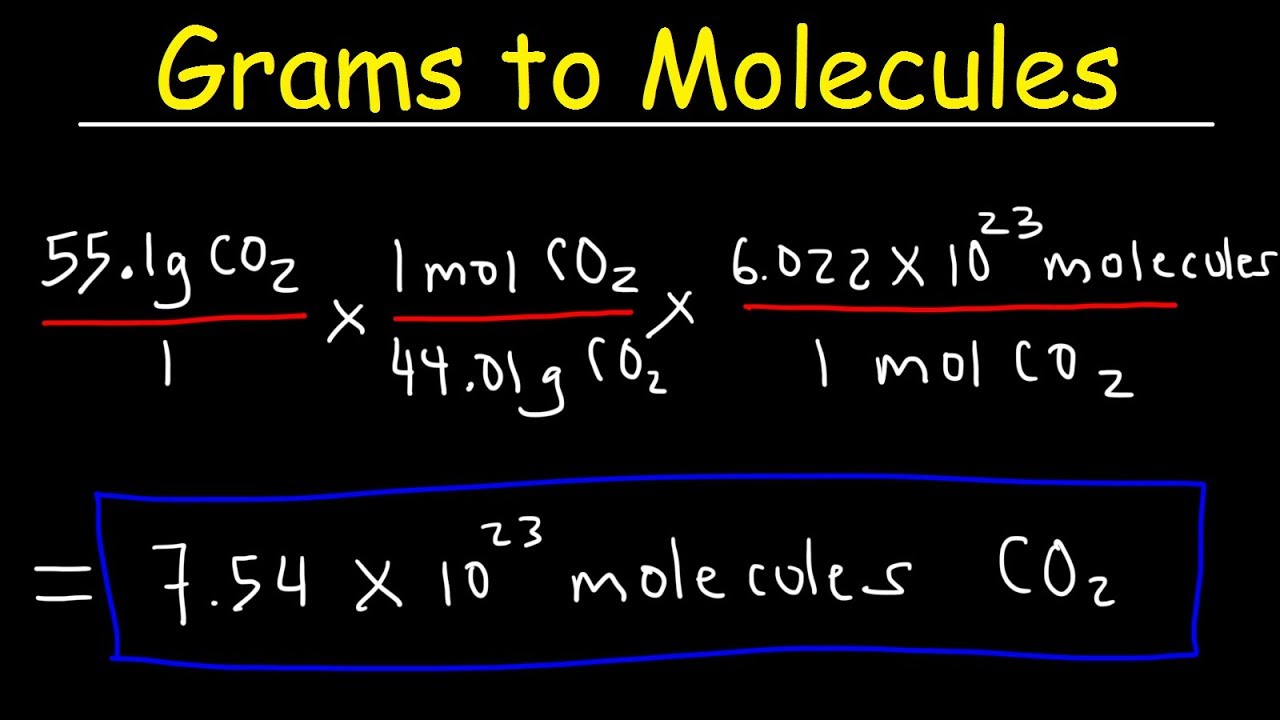 Grams To Molecules And Molecules To Grams Conversion Youtube From youtube.com
Grams To Molecules And Molecules To Grams Conversion Youtube From youtube.com
Here we know that 1 mole of H. A molar mass 602 x 1023 molecules and for a gas 224 liters at STP. Mole-Mass given moles and converting to mass 2 conversion factors 3. Watch video circle and label what you are given and what you are trying to find. Al — 037062 2 018531 O 3 — 039586 1 039586 Al is the limiting reagent 4 Determine moles of product formed. When going from molecules to moles you divide by 602 x 10 23.
Then knowing that there are 6022x1023 molecules in one mol Avogadros number you can find the number of molecules in however many mol of the substance that you have.
We can use this information to convert grams to molecules or liters molecules to grams or liters or liters to grams or molecules. In this video we go over how t. When going from molecules to moles you divide by 602 x 10 23. Al to Al 2 O. Mass-Mole given mass and converting to moles 2 conversion factors 4. Here we know that 1 mole of.
 Source: pinterest.com
Source: pinterest.com
Students will be able to convert between grams of the various substances in a chemical reaction. Here we know that 1 mole of H. It shows how to find molar mass and it shows how to convert from grams to molecules and vice versa. See explanation to convert grams to moles you will need to molar mass of that specific element molar mass is found my multiplying the number of atoms present in the compound by the relative atomic mass found on the bottom of the. Students will be able to convert between grams of the various substances in a chemical reaction.
 Source: youtube.com
Source: youtube.com
This is the currently selected item. When going from moles to molecules you multiply by 602 x 10 23. First the molar mass allows you to change mass into mol. 2 Convert grams to moles. Watch video circle and label what you are given and what you are trying to find.
 Source: pinterest.com
Source: pinterest.com
Convert 40 moles to molecules. When going from molecules to moles you divide by 602 x 10 23. Then knowing that there are 6022x1023 molecules in one mol Avogadros number you can find the number of molecules in however many mol of the substance that you have. When going from moles to molecules you multiply by 602 x 10 23. A molar mass 602 x 1023 molecules and for a gas 224 liters at STP.
 Source: gr.pinterest.com
Source: gr.pinterest.com
It teaches the basic concepts of Avogadros Number and stoichiometry. It also helps them remember the values of the mole by using an avocado. Watch video circle and label what you are given and what you are trying to find. When going from moles to molecules you multiply by 602 x 10 23. Practice converting moles to grams and from grams to moles when given the molecular weight.
 Source: youtube.com
Source: youtube.com
In this stage of the course you are most likely dealing with the 4 basic types of stoichiometry problems 1. The equation is 2 H3PO4 3 CaOH2 Ca3PO4 2 6 H2O. Al — 037062 2 018531 O 3 — 039586 1 039586 Al is the limiting reagent 4 Determine moles of product formed. Here we know that 1 mole of H. 6 2 g r a m s H X 2 1 g r a m O X.
 Source: youtube.com
Source: youtube.com
To convert between moles and molecules you need to remember that one mole of any substance contains 602 x 10 23 particles eg atoms or molecules. Converting moles and mass. Students will be able to convert between grams of the various substances in a chemical reaction. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. We can use this information to convert grams to molecules or liters molecules to grams or liters or liters to grams or molecules.
 Source: pinterest.com
Source: pinterest.com
How To Do Stoichiometry Grams To Moles. It teaches the basic concepts of Avogadros Number and stoichiometry. 2 NaCl 2 Na Cl 2 1. Watch video circle and label what you are given and what you are trying to find. Watch video circle and label what you are given and what you are trying to find.
 Source: youtube.com
Source: youtube.com
A molar mass 602 x 1023 molecules and for a gas 224 liters at STP. The equation is 2 H3PO4 3 CaOH2 Ca3PO4 2 6 H2O. See explanation to convert grams to moles you will need to molar mass of that specific element molar mass is found my multiplying the number of atoms present in the compound by the relative atomic mass found on the bottom of the. Or in plain English two moles of hydrogen and one mole of oxygen react to produce two moles of water. Converting moles and mass.
 Source: pinterest.com
Source: pinterest.com
35 moles H 2 O x 1802 grams1 mole H 2 O 35 x 1802 grams 6307 grams Check to see if you answer makes sense. Now lets use equation 1 and multiply each side by 1 gram s as we can do in algebra. Using the molar mass as a conversion factor you can calculate the number of moles present in the stated number of grams of the species. The equation is 2 H3PO4 3 CaOH2 Ca3PO4 2 6 H2O. Take a look at the example of how to calculate moles from grams.
 Source: pinterest.com
Source: pinterest.com
When going from moles to molecules you multiply by 602 x 10 23. Using the molar mass as a conversion factor you can calculate the number of moles present in the stated number of grams of the species. Converting moles and mass. How To Do Stoichiometry Grams To Moles. How many grams of sodium chloride are required to make.
 Source: pinterest.com
Source: pinterest.com
Or in plain English two moles of hydrogen and one mole of oxygen react to produce two moles of water. 2 Convert grams to moles. Take a look at the example of how to calculate moles from grams. Relating reaction stoichiometry and the ideal gas law. Or in plain English two moles of hydrogen and one mole of oxygen react to produce two moles of water.
 Source: pinterest.com
Source: pinterest.com
2 NaCl 2 Na Cl 2 1. To convert between moles and molecules you need to remember that one mole of any substance contains 602 x 10 23 particles eg atoms or molecules. Relating reaction stoichiometry and the ideal gas law. How many grams of sodium chloride are required to make. Mole-Mass given moles and converting to mass 2 conversion factors 3.
 Source: youtube.com
Source: youtube.com
Mole-Mole given moles and converting to moles 1 conversion factor 2. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. Then knowing that there are 6022x1023 molecules in one mol Avogadros number you can find the number of molecules in however many mol of the substance that you have. 2 Convert grams to moles. Practice converting moles to grams and from grams to moles when given the molecular weight.
 Source: pinterest.com
Source: pinterest.com
When going from molecules to moles you divide by 602 x 10 23. 2 NaCl 2 Na Cl 2 1. Now lets use equation 1 and multiply each side by 1 gram s as we can do in algebra. Al — 037062 2 018531 O 3 — 039586 1 039586 Al is the limiting reagent 4 Determine moles of product formed. Here we know that 1 mole of H.
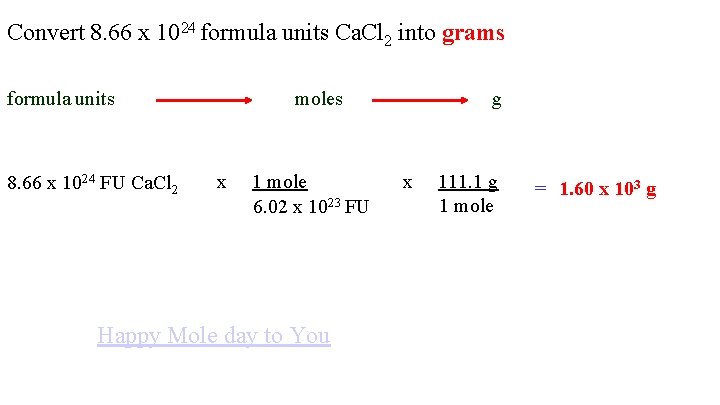 Source: slidetodoc.com
Source: slidetodoc.com
We can use this information to convert grams to molecules or liters molecules to grams or liters or liters to grams or molecules. Convert 40 moles to molecules. CHEMISTRY WORKSHEET 6 MIXED MOLE PROBLEMS GRAMS MOLECULES AND LITERS You now know three things a mole can be. Mole-Mass given moles and converting to mass 2 conversion factors 3. This is the currently selected item.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to convert grams to molecules in stoichiometry by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






