Your How to convert from grams to moles images are ready. How to convert from grams to moles are a topic that is being searched for and liked by netizens now. You can Download the How to convert from grams to moles files here. Get all royalty-free images.
If you’re searching for how to convert from grams to moles pictures information connected with to the how to convert from grams to moles keyword, you have come to the ideal blog. Our site frequently provides you with hints for seeking the maximum quality video and picture content, please kindly surf and find more enlightening video articles and graphics that fit your interests.
How To Convert From Grams To Moles. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Grams Moles x Molar Mass. N m M. After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive.
 Grams To Moles Calculator Plus Convert Moles To Grams In 2021 Grams To Moles Molar Mass Mass To Moles From pinterest.com
Grams To Moles Calculator Plus Convert Moles To Grams In 2021 Grams To Moles Molar Mass Mass To Moles From pinterest.com
Number of moles Weight of compound in grams molecular weight of compound In our example the weight of NaCl is 100 grams and its molecular weight is 5852 gmoles. You can also verify the results by putting the values in free grams to molecules calculator. A gramg is a metric unit of mass and was formally defined as the absolute weight of a volume of pure water equal to the cube of the hundredth part of a meter and at the temperature of melting ice. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Formula to convert moles to grams. Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is.
Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is.
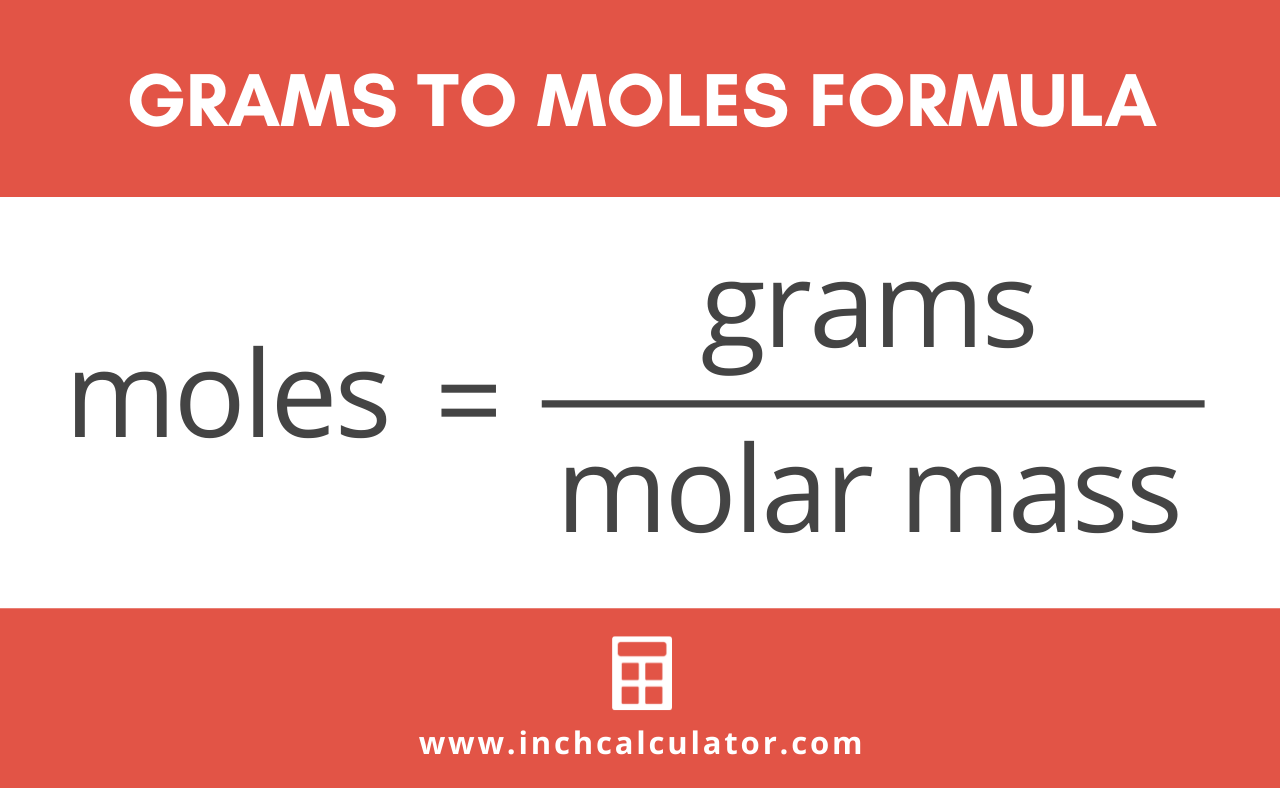
Do a quick conversion. To convert grams to moles multiply the number of grams by 1 molemolar mass. You can also verify the results by putting the values in free grams to molecules calculator. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. The atomic mass of rubidium is 854678. Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is.
 Source: pinterest.com
Source: pinterest.com
The atomic mass of rubidium is 854678. How to go from moles to grams. The mass has a unit of grams and kilograms and the number of moles has a unit of moles. Likewise to convert moles to grams multiply the number of moles by molar mass1 mole. Number of moles Weight of compound in grams molecular weight of compound In our example the weight of NaCl is 100 grams and its molecular weight is 5852 gmoles.
 Source: pinterest.com
Source: pinterest.com
Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is. To convert grams to moles multiply the number of grams by 1 molemolar mass. This dimensional analysis video tuto. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. While a mole is the unit of measurement for amount of substance in the International System of Units.
 Source: pinterest.com
Source: pinterest.com
You can also verify the results by putting the values in free grams to molecules calculator. Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is. N 5000 90075. 0700 mole x 340146 gramsmole 238 grams the answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures in. Formula to convert grams to moles.
 Source: pinterest.com
Source: pinterest.com
To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. Before we begin note that rubidium Rb is a chemical element that you can find in the periodic table and grams g is a metric measurement. Divide the number of grams of the compound NaOH by the molecular weight and as a result the grams g unit cancels and all we have left is. That means that one mole of rubidium weighs 854678 grams 854678 gramsmoles.
 Source: pinterest.com
Source: pinterest.com
You can also verify the results by putting the values in free grams to molecules calculator. N 55503 m o l e s. This dimensional analysis video tuto. See an example of converting grams to moles. Number of moles Weight of compound in grams molecular weight of compound In our example the weight of NaCl is 100 grams and its molecular weight is 5852 gmoles.
 Source: hu.pinterest.com
Source: hu.pinterest.com
To convert grams to moles multiply the number of grams by 1 molemolar mass. Do a quick conversion. The atomic mass of rubidium is 854678. This dimensional analysis video tuto. Before we begin note that rubidium Rb is a chemical element that you can find in the periodic table and grams g is a metric measurement.
 Source: pinterest.com
Source: pinterest.com
Formula to convert grams to moles. To convert grams to moles multiply the number of grams by 1 molemolar mass. The mass has a unit of grams and kilograms and the number of moles has a unit of moles. Thus the number of moles in the given sample of. You can also verify the results by putting the values in free grams to molecules calculator.
 Source: pinterest.com
Source: pinterest.com
Both properties are related to each other using molar mass which measures the mass of a. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. How to go from moles to grams. N m M where.
 Source: pinterest.com
Source: pinterest.com
You can also verify the results by putting the values in free grams to molecules calculator. That means that one mole of rubidium weighs 854678 grams 854678 gramsmoles. After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive. To convert grams to moles multiply the number of grams by 1 molemolar mass. Both properties are related to each other using molar mass which measures the mass of a.
 Source: br.pinterest.com
Source: br.pinterest.com
Before we begin note that rubidium Rb is a chemical element that you can find in the periodic table and grams g is a metric measurement. 0700 mole x 340146 gramsmole 238 grams the answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures in. The answer will be the number of moles of the compound. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. This dimensional analysis video tuto.
 Source: pinterest.com
Source: pinterest.com
How to go from moles to grams. Likewise to convert moles to grams multiply the number of moles by molar mass1 mole. How to Convert Grams to Moles. To convert grams to moles multiply the number of grams by 1 molemolar mass. Do a quick conversion.
 Source: pinterest.com
Source: pinterest.com
Carrying out grams to moles conversion. You can also verify the results by putting the values in free grams to molecules calculator. While a mole is the unit of measurement for amount of substance in the International System of Units. Do a quick conversion. 7 moles in to grams 803726 gramsBased on that information to convert 1009 moles of curium to grams we multiply 1009 moles of curium by 247.
 Source: pinterest.com
Source: pinterest.com
How to go from moles to grams. The atomic mass of rubidium is 854678. Carrying out grams to moles conversion. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. Grams Moles x Molar Mass.
 Source: pinterest.com
Source: pinterest.com
Likewise to convert moles to grams multiply the number of moles by molar mass1 mole. Thus the number of moles in the given sample of. N 5000 90075. Both properties are related to each other using molar mass which measures the mass of a. How to go from moles to grams.
 Source: pinterest.com
Source: pinterest.com
How to Convert Grams to Moles. A gramg is a metric unit of mass and was formally defined as the absolute weight of a volume of pure water equal to the cube of the hundredth part of a meter and at the temperature of melting ice. N 5000 90075. Carrying out grams to moles conversion. Convert 02 moles of Sodium chloride.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to convert from grams to moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.