Your How to change mole into grams images are ready. How to change mole into grams are a topic that is being searched for and liked by netizens now. You can Get the How to change mole into grams files here. Get all royalty-free photos and vectors.
If you’re looking for how to change mole into grams pictures information linked to the how to change mole into grams keyword, you have visit the ideal site. Our site always gives you suggestions for seeking the maximum quality video and picture content, please kindly surf and locate more informative video articles and images that match your interests.
How To Change Mole Into Grams. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. This will give you the molecular mass of the molecule. One mole consists of Avogadro number of atoms. 8 moles In to grams 918544 grams.
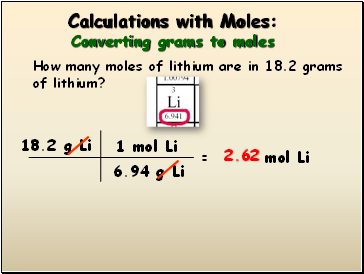 Hubert Hudson Fold Frog Convert Moles To Grams Calculator Uctsc Org From uctsc.org
Hubert Hudson Fold Frog Convert Moles To Grams Calculator Uctsc Org From uctsc.org
Do a quick conversion. This will give you the molecular mass of the molecule. How do you convert moles per liter into grams. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Multiply both the values. Thus the conversion of moles to grams formula is.
A mole of a substance is defined as the mass of substance containing the same number of fundamental units as there are atoms in exactly 12 grams of Carbon-12 which is approximately 6022 x 10 23 atoms.
10 moles In to grams 114818 grams. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. X 122550 g 250 mol 1 mol. To convert moles into grams determine the number of moles preset and the molar mass of the compound. Calculate how many moles are mentioned in the question. Use this web page to learn to convert between moles In and gram.
 Source: slideplayer.com
Source: slideplayer.com
If you know the quantity of mole it can be converted into grams and vice versa. Calculate how many moles are mentioned in the question. How do you convert moles per liter into grams. Moles gramsmols Lets look at the units. Here we will show you how to convert 8708 moles of carbon to grams.
 Source: uctsc.org
Source: uctsc.org
The formula for moles to grams is given by. Before we delve deeper into the grams to moles conversion calculation procedure lets do a quick recap of the basics. You can convert moles per litre into grams per litre by multiplying the value in grams per litre by. Its easier to work with grams so convert the mass. 9 moles In to grams 1033362 grams.
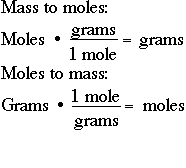 Source: socratic.org
Source: socratic.org
How do you convert moles per liter into grams. Aug 04 2015 To transform moles into grams decide the variety of moles preset and the molar mass of the compound. 1 moles In to grams 114818 grams. Moles gramsmols Lets look at the units. After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive.
 Source: wikihow.com
Source: wikihow.com
After about a semester and a half I had forgotten how to convert from grams to molesThis article was extremely clear precise and definitive. How do you change particles to grams and grams to particles. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. Grams Moles x Molar Mass. To convert the moles into grams multiply the mass of the substance by the molecular weight formula weight.
 Source: no.pinterest.com
Source: no.pinterest.com
To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. Where is the molar mass of the substance. 6 moles In to grams 688908 grams. One mole consists of Avogadro number of atoms.
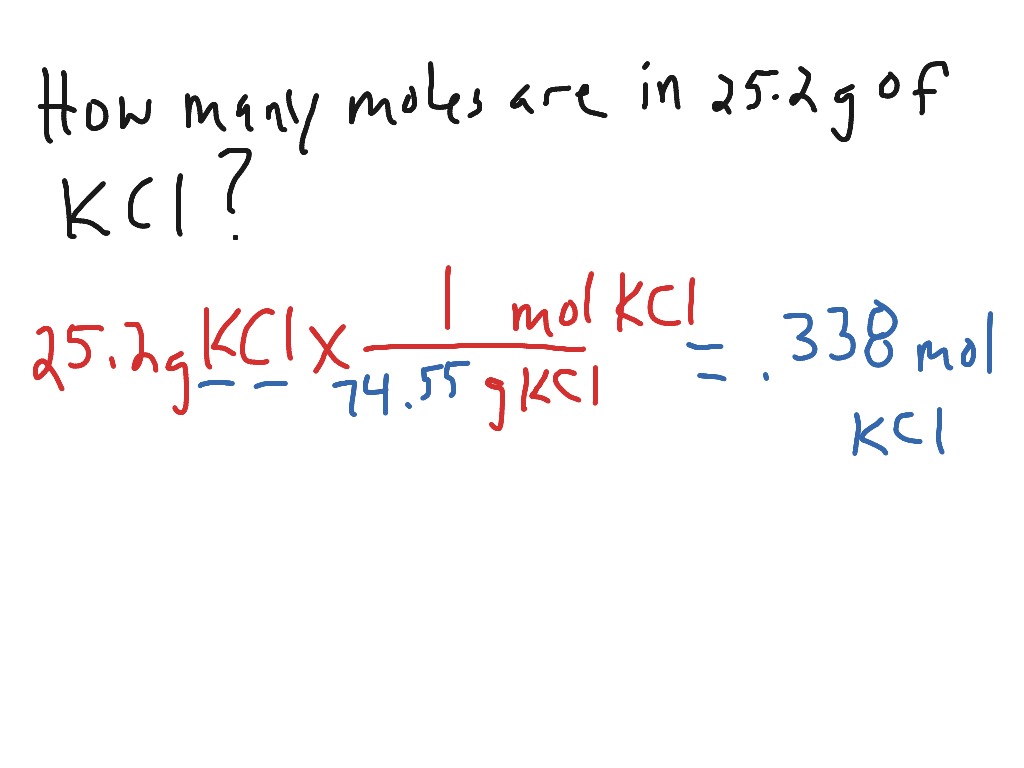 Source: showme.com
Source: showme.com
This dimensional analysis video tuto. More commonly written for this application as. This dimensional analysis video tuto. You can convert moles per litre into grams per litre by multiplying the value in grams per litre by. Aug 04 2015 To transform moles into grams decide the variety of moles preset and the molar mass of the compound.
 Source: youtube.com
Source: youtube.com
As the correct answer. Thus the conversion of moles to grams formula is. The SI base unit for quantity of substance is the mole. To convert moles into grams determine the number of moles preset and the molar mass of the compound. One mole consists of Avogadro number of atoms.
 Source: pinterest.com
Source: pinterest.com
Set the quantity to 2000 moles of carbon mol C then press Begin. One mole consists of Avogadro number of atoms. 4 If this problem were set up like the proportion above you would have this. Add these values together for each different atom in the molecule. More commonly written for this application as.
 Source: khanacademy.org
Source: khanacademy.org
You have three steps to convert mole values to grams. 3 moles In to grams 344454 grams. 1 moles In to grams 114818 grams. You have three steps to convert mole values to grams. Since moles grams of compoundthe molar mass to find the mass you will need to do mass molar mass x molarity.
 Source: tr.pinterest.com
Source: tr.pinterest.com
The answer will be the number of moles of the compound. Grams Moles x Molar Mass. Its easier to work with grams so convert the mass. 1 moles Urea 6005526 gram using the molecular weight calculator and the molar mass of CONH22. 21008 3206 418 106076.
 Source: uctsc.org
Source: uctsc.org
5988 kg 5988 g. One mole consists of Avogadro number of atoms. Find the molar mass of the substance. Moles gramsmols Lets look at the units. Set the quantity to 2000 moles of carbon mol C then press Begin.
 Source: youtube.com
Source: youtube.com
This chemistry video tutorial explains how to convert moles to grams which is useful in typical stoichiometry problems. 4 If this problem were set up like the proportion above you would have this. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. How do you convert moles per liter into grams. 7 moles In to grams 803726 grams.
 Source: pinterest.com
Source: pinterest.com
Here we will show you how to convert 8708 moles of carbon to grams. You have three steps to convert mole values to grams. 10 moles In to grams 114818 grams. The formula for moles to grams is given by. 1 moles Urea 6005526 gram using the molecular weight calculator and the molar mass of CONH22.
 Source: study.com
Source: study.com
Find the molar mass of the substance. This will give you the molecular mass of the molecule. Multiply both the values. Calculate how many moles are mentioned in the question. One mole consists of Avogadro number of atoms.
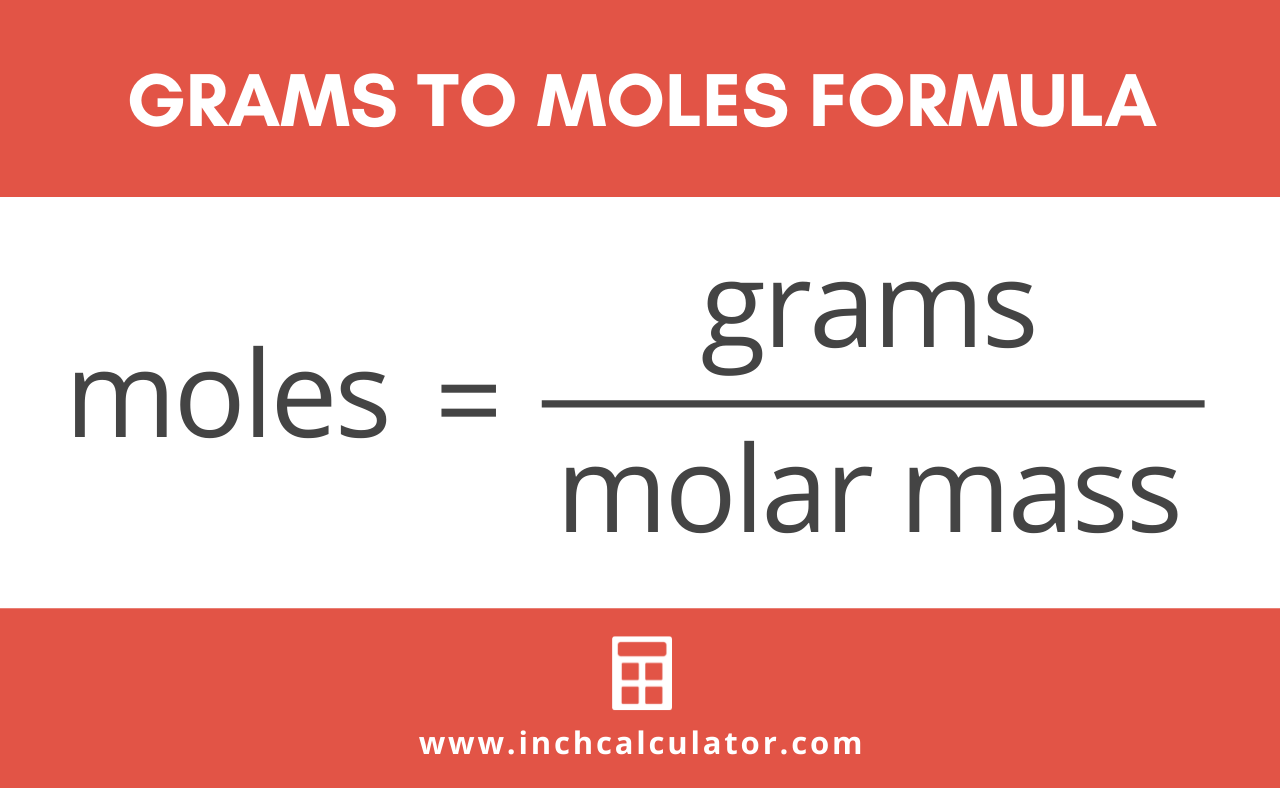 Source: uctsc.org
Source: uctsc.org
As the correct answer. To convert moles into grams determine the number of moles preset and the molar mass of the compound. In other words it is the product of the mass of the substance and its molecular weight. Aug 04 2015 To transform moles into grams decide the variety of moles preset and the molar mass of the compound. If you know the quantity of mole it can be converted into grams and vice versa.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to change mole into grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






