Your How to change molar mass to molecular mass images are ready in this website. How to change molar mass to molecular mass are a topic that is being searched for and liked by netizens today. You can Get the How to change molar mass to molecular mass files here. Get all royalty-free photos and vectors.
If you’re looking for how to change molar mass to molecular mass images information related to the how to change molar mass to molecular mass topic, you have visit the ideal blog. Our website frequently provides you with suggestions for downloading the maximum quality video and picture content, please kindly surf and find more enlightening video articles and graphics that match your interests.
How To Change Molar Mass To Molecular Mass. Its units are molL moldm 3 or molm 3. To prepare 1 L of 05 M sodium chloride solution then as per the formula use 2922 g of sodium chloride 05 molL 1L 5844 gmol. In this method we find a known volume of a gas at STP. The molecular weight is unitless but is given.
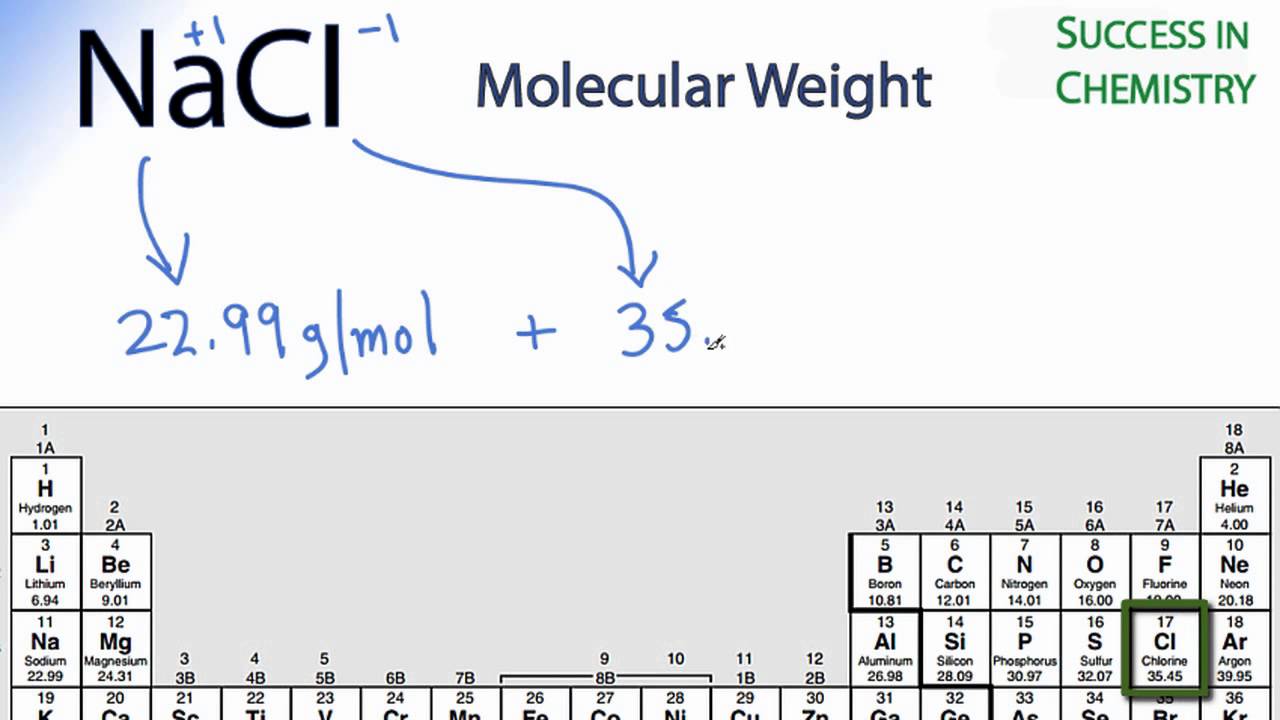 Nacl Molar Mass Molecular Weight Youtube From youtube.com
Nacl Molar Mass Molecular Weight Youtube From youtube.com
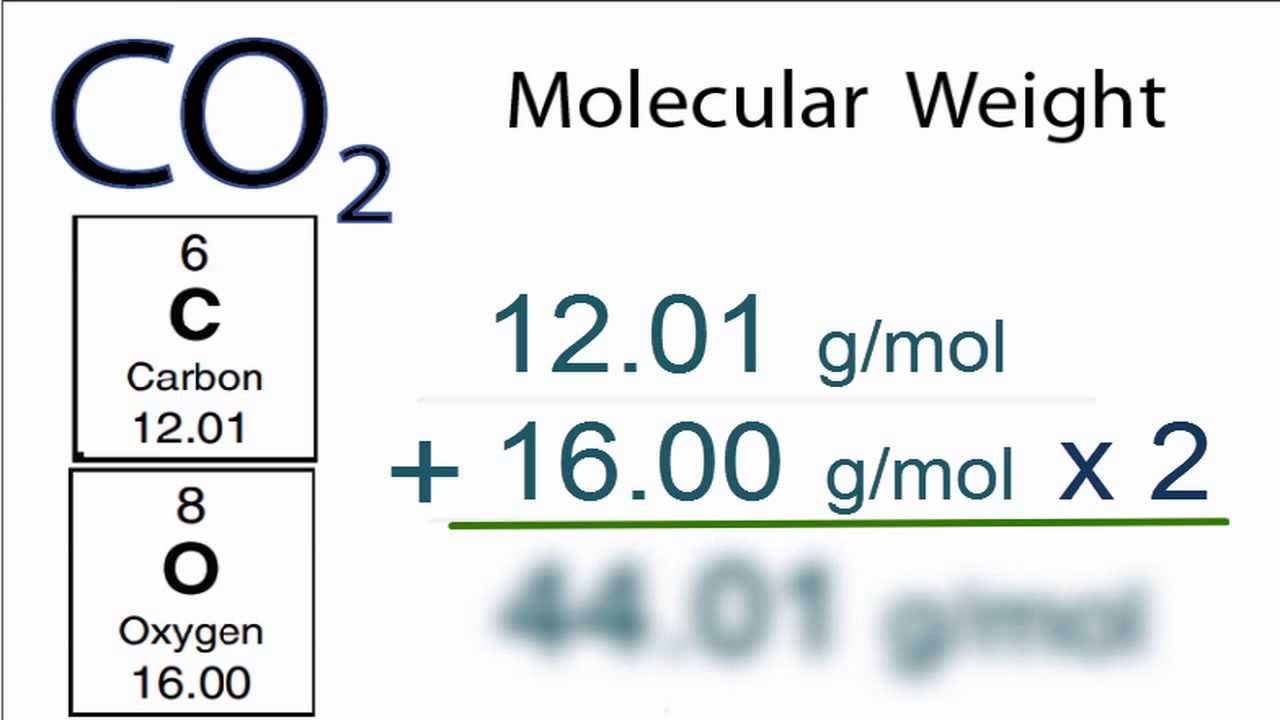
It is 5844 g mol 1. Molar mass of water is 1801528 000044 gmol The molar mass of h 2 o is 1802 gmol. Pricing Info Sample Submission Guidelines Submit Your Samples. The molecular weight is unitless but is given. Thus by knowing the molar mass we can determine the number of moles contained in a given mass of a sample. Divide the molar mass for the molecular formula by the empirical formula mass.
MGE Innovation Center 505 South Rosa Road Suite 238 Madison WI 53719.
Convert 02 moles of Sodium chloride. You can use Molar mass of the substance alone to calculate molar mass. It is 5844 g mol 1. Molecular Weight to Molarity. The molar mass of KClO3 is 122548 gmol. This chemistry video tutorial explains how to calculate the molar mass of a compound.
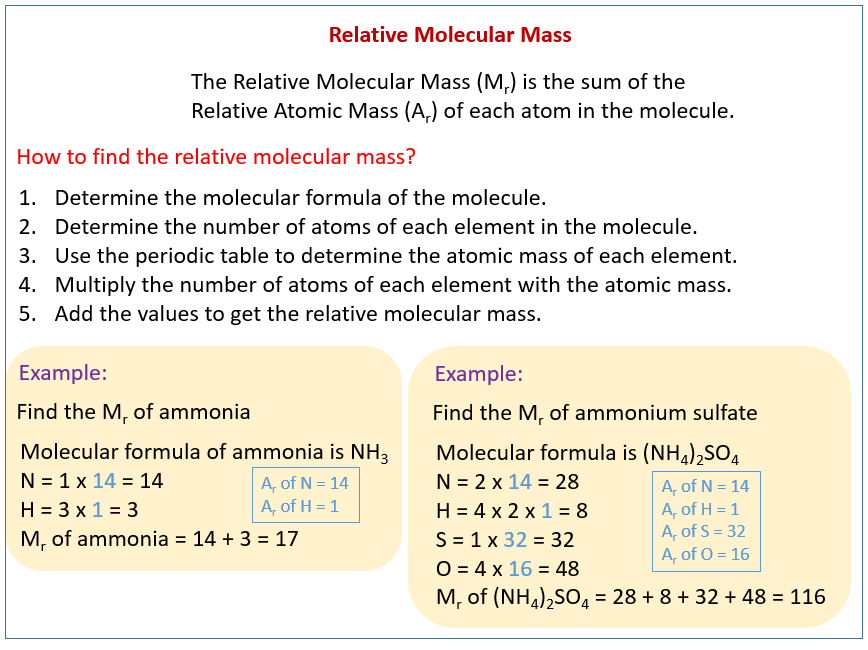 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Molar mass of water is 1801528 000044 gmol The molar mass of h 2 o is 1802 gmol. As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms. The molar mass links the mass of a substance to its moles. The molecular weight of this compound is 18018gmol. The mole was defined in such a way that the molar mass of a compound in gmol is numerically equal for all practical purposes to the average mass of one molecule in daltons.
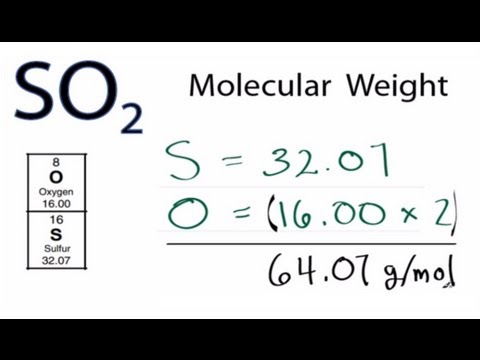 Source: youtube.com
Source: youtube.com
Let me make it more clear with an example of sodium chloride. Molar mass is the mass of a mole of a particular substance. It is 5844 g mol 1. For example the molar mass of NaCl can be calculated for finding the atomic mass of sodium 2299 gmol and the atomic mass of chlorine 3545 gmol and combining them. This chemistry video tutorial explains how to calculate the molar mass of a compound.
 Source: pinterest.com
Source: pinterest.com
Difference Between Molar Mass and Molecular Weight Definition. For example the molar mass of NaCl can be calculated for finding the atomic mass of sodium 2299 gmol and the atomic mass of chlorine 3545 gmol and combining them. Molar Mass Molecular Weight - The term mole also referred to as mol was first used by Ostwald in 1896. It is 5844 g mol 1. Determine the molar mass from the mass of the unknown and the number of moles of unknown.
 Source: pinterest.com
Source: pinterest.com
To calculate the molar mass of a compound with multiple atoms sum all the atomic mass of the constituent atoms. Mary filled her 590 L metal cylinder with 1066 g of gas to a pressure of 2025 psi and at a temperature of 25 C. This can be calculated from the molecular weights and mole fractions of the components. Thus by knowing the molar mass we can determine the number of moles contained in a given mass of a sample. The mole was defined in such a way that the molar mass of a compound in gmol is numerically equal for all practical purposes to the average mass of one molecule in daltons.
 Source: pinterest.com
Source: pinterest.com
Let me make it more clear with an example of sodium chloride. In order to convert the mass and molar flow rates of the entire solution we need to know the average molecular weight of the solution. Convert 02 moles of Sodium chloride. The molecular weight of this compound is 18018gmol. Gram Molecular Mass or Molar Mass.
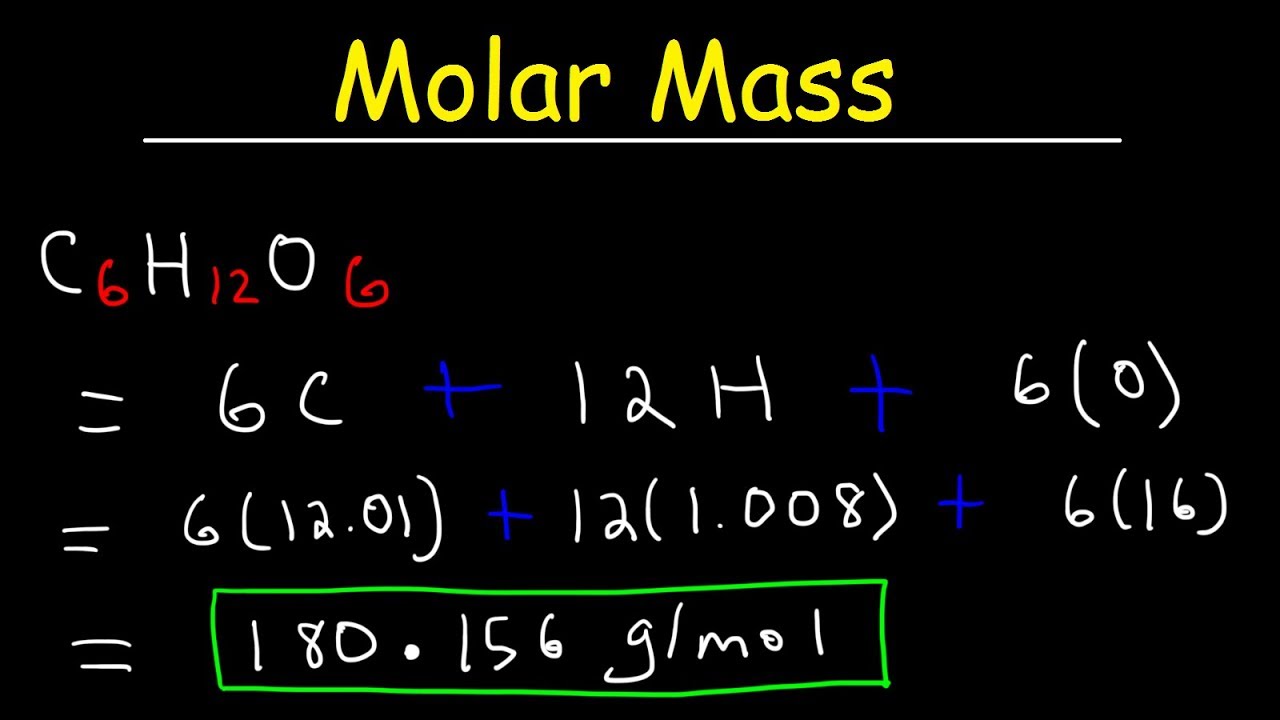 Source: youtube.com
Source: youtube.com
Its units are molL moldm 3 or molm 3. In order to convert the mass and molar flow rates of the entire solution we need to know the average molecular weight of the solution. So we can write down an equation expressing the molar mass as a function of x. The molar mass of a substance is the mass in grams of 1 mole of the substance. Calculate the molar mass of this gas and use the value of the molar mass to identify the gas.
 Source: khanacademy.org
Source: khanacademy.org
Molar mass grams moles so we need to find the grams and divide that by the number of moles. The number of grams of KClO3 will be 30637. Grams Moles x Molar Mass. Molar Mass Molecular Weight - The term mole also referred to as mol was first used by Ostwald in 1896. For example the molar mass of NaCl can be calculated for finding the atomic mass of sodium 2299 gmol and the atomic mass of chlorine 3545 gmol and combining them.
 Source: pinterest.com
Source: pinterest.com
The molar mass of any substance can. It contains plenty of examples and practice problemsMy E-Book. To calculate the molar mass of a compound with multiple atoms sum all the atomic mass of the constituent atoms. Molar Mass Molecular Weight - The term mole also referred to as mol was first used by Ostwald in 1896. Finding molar mass also called molecular weight molecular mass and gram formula mass is an essential skill in chemistry especially for mole to gram conv.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
You can use Molar mass of the substance alone to calculate molar mass. Mass of 1 mole of oxygen is 159994 grams. Molar mass of water is 1801528 000044 gmol The molar mass of h 2 o is 1802 gmol. Molecular Weight to Molarity. The molecular mass expressed in grams is called gram molecular mass GMM Method I Molar Volume Method Principle.
 Source: youtube.com
Source: youtube.com
Molar concentration is the amount of a solute present in one unit of a solution. This can be calculated from the molecular weights and mole fractions of the components. Molecular mass 40078 x 3 3097361 x 2 159994 x 8 molecular mass 120234 6194722 1279952 molecular mass 31017642 from. We know the molecular formula is Cx H 2x O x. To prepare 1 L of 05 M sodium chloride solution then as per the formula use 2922 g of sodium chloride 05 molL 1L 5844 gmol.
 Source: youtube.com
Source: youtube.com
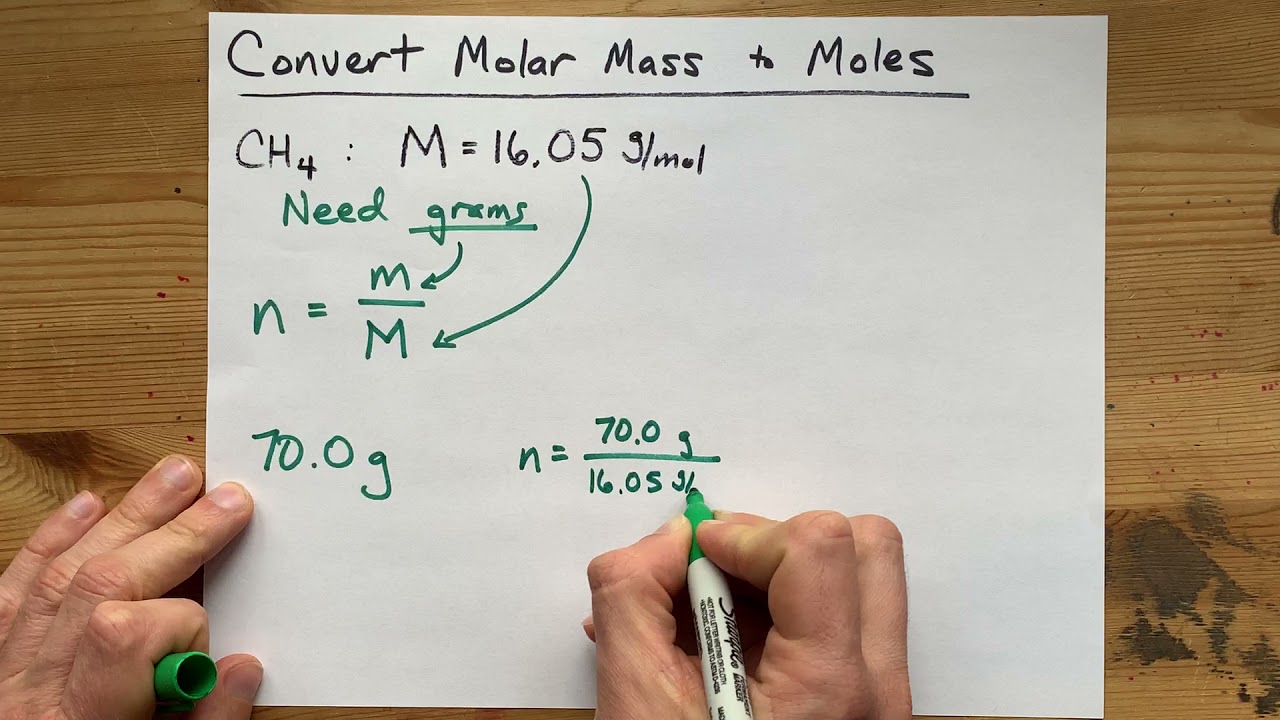
Molar mass of water is 1801528 000044 gmol The molar mass of h 2 o is 1802 gmol. Set up this equation and place the grams on top. Determine the moles of unknown the solute from the molality of the solution and the mass of solvent in kilograms used to make the solution. Divide the molar mass for the molecular formula by the empirical formula mass. The unit of molar mass is kgmol-1 or kgmol.
 Source: pinterest.com
Source: pinterest.com
This chemistry video tutorial explains how to calculate the molar mass of a compound. Calculate the molar mass of this gas and use the value of the molar mass to identify the gas. It contains plenty of examples and practice problemsMy E-Book. The result determines how many times to multiply the subscripts in the empirical formula to get the molecular formula. So we can write down an equation expressing the molar mass as a function of x.
 Source: youtube.com
Source: youtube.com
Find out the molar mass of the substance hint. The mass in g of 1 mole of a substance is known as the molar mass or molecular weight of the substance. It contains plenty of examples and practice problemsMy E-Book. Determine the molar mass from the mass of the unknown and the number of moles of unknown. Lets now apply equation 3 and 4 to solve the following problem.
 Source: pinterest.com
Source: pinterest.com
Multiply the given number of moles 250 mol by the molar mass 122548 gmol to get the grams. Therefore the molar mass 159994 gmol. In order to convert the mass and molar flow rates of the entire solution we need to know the average molecular weight of the solution. The term molecular weight can be defined as the mass of a molecule. Convert 02 moles of Sodium chloride.
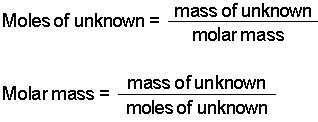 Source: chem.purdue.edu
Source: chem.purdue.edu
And using the concept that one mole of every gas occupies 224 dm 3 by volume we calculate the molar mass of the gas. You can use Molar mass of the substance alone to calculate molar mass. Molar flow rates and mass flow rates are related by the molecular weight also known as the molar mass of the solution. The molar mass of any substance can. Multiply the given number of moles 250 mol by the molar mass 122548 gmol to get the grams.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to change molar mass to molecular mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






