Your How to change mass to moles images are ready. How to change mass to moles are a topic that is being searched for and liked by netizens today. You can Find and Download the How to change mass to moles files here. Find and Download all free photos and vectors.
If you’re looking for how to change mass to moles pictures information related to the how to change mass to moles interest, you have visit the right blog. Our website frequently provides you with suggestions for viewing the maximum quality video and image content, please kindly search and find more enlightening video articles and images that match your interests.
How To Change Mass To Moles. How To Calculate The Quantity Of Moles Multiply wv by. The unit is typically gmol. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight. But if we want to express it in KgMole then devide it by 1000.
 How To Convert Grams To Moles Video Lesson Transcript Study Com From study.com
How To Convert Grams To Moles Video Lesson Transcript Study Com From study.com
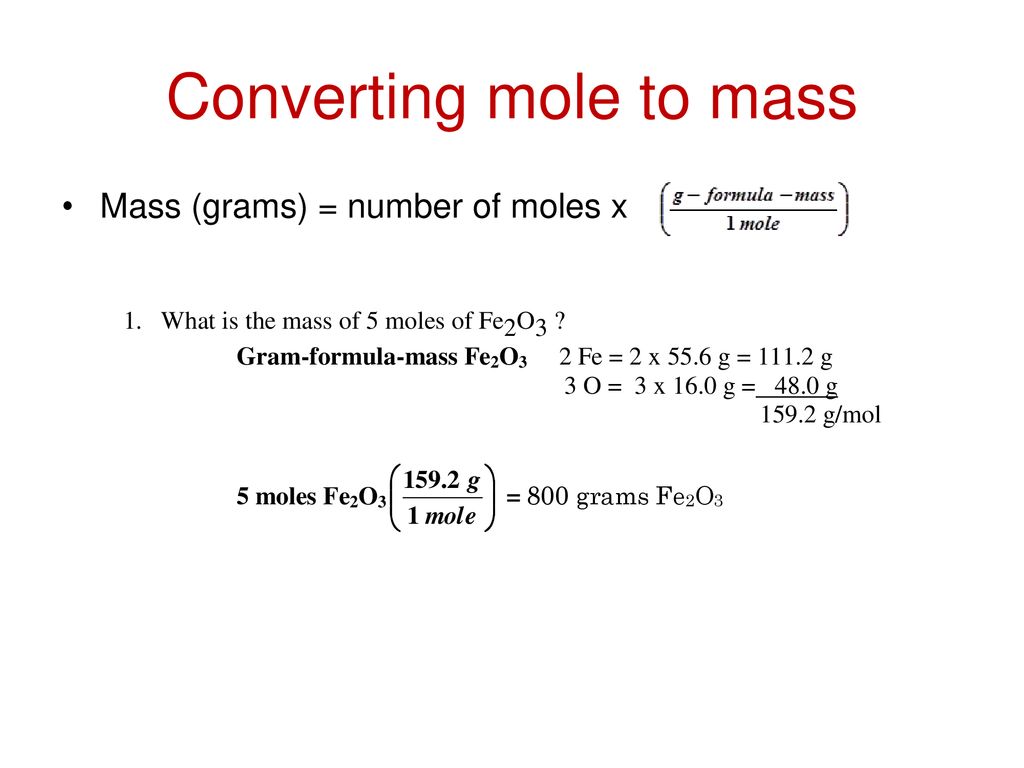
Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. Grams Moles x Molar Mass. The formula for moles to grams is given by. 0100 L of 025 M NH4Cl 0050 L of 025 M NaOH. But if we want to express it in KgMole then devide it by 1000. Convert 02 moles of Sodium chloride.
The mole is the SI unit of the measurement for the amount of a substance.
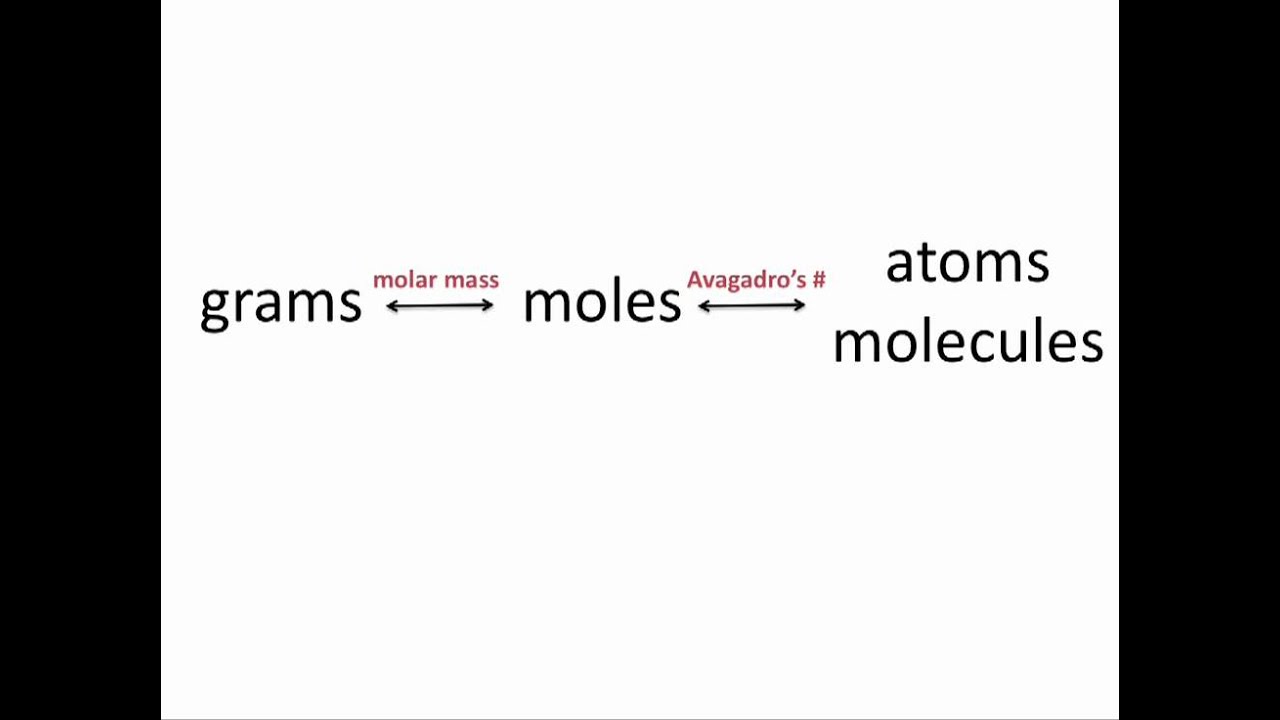
Or multiply it by 10 -3 ten to the power -3. How To Calculate The Quantity Of Moles Multiply wv by. While a mole is the unit of measurement for amount of substance in the International System of Units. Molar mass of water is 18 gramsmole. Converting grams to moles involves 2 steps. Finding molar mass starts with units of grams per mole gmol.
 Source: slideplayer.com
Source: slideplayer.com
Find the molecular mass of the compound. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. Easy methods to change molar mass to moles. Where is the molar mass of the substance. Grams Moles x Molar Mass.
 Source: study.com
Source: study.com
Moles of HCl 20365 547 moles of HCl Moles of water 8018 444 moles of H2O. Moles to Grams Conversion Formula. Where is the molar mass of the substance. THE QUESTIONS ask to compute how many moles of base it takes to change the pH of this buffered solution by 010 pH unit. Molar mass is generally expressed in gramsmole.
 Source: youtube.com
Source: youtube.com
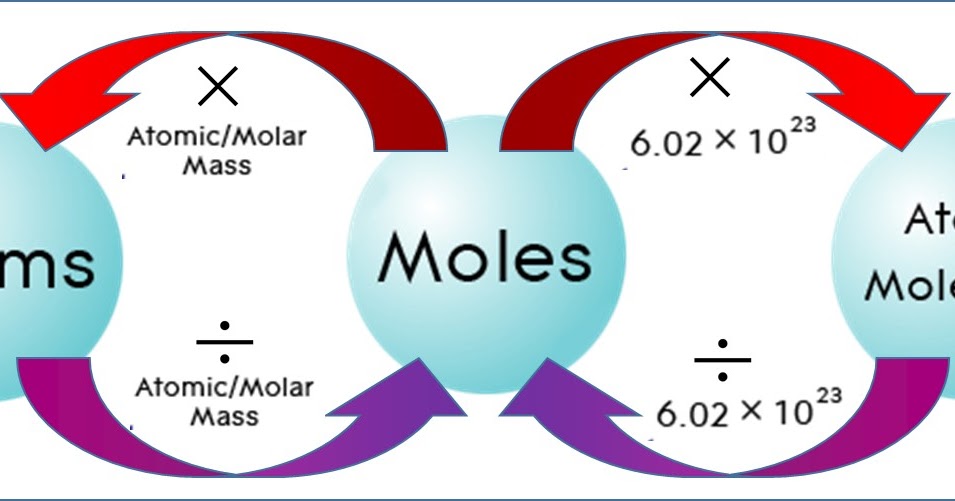
Molar mass of Oxygen is 32 gram mole. Or multiply it by 10 -3 ten to the power -3. Grams Moles x Molar Mass. If you know the quantity of mole it can be converted into grams and vice versa. Find the molecular mass of the compound.
 Source: saylordotorg.github.io
Source: saylordotorg.github.io
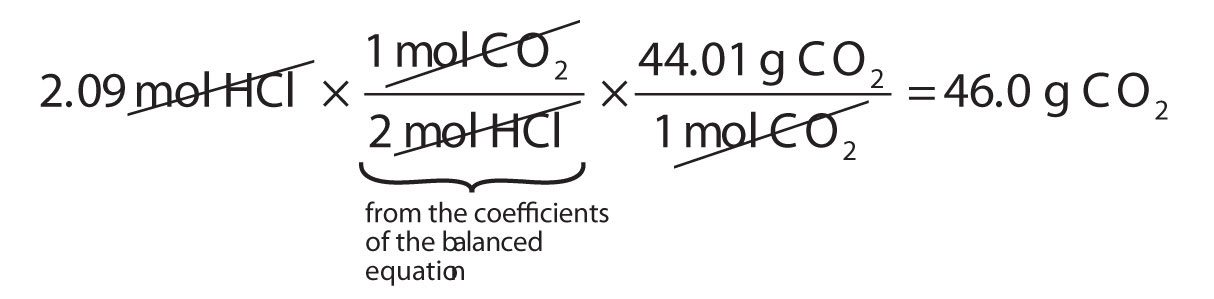
Solution The solution contains 20 grams of HCL acid and 80 grams of water. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. How To Calculate The Quantity Of Moles Multiply wv by. To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. THE QUESTIONS ask to compute how many moles of base it takes to change the pH of this buffered solution by 010 pH unit.
 Source: slideplayer.com
Source: slideplayer.com
Converting grams to moles involves 2 steps. Where is the molar mass of the substance. Substitute the identified values to calculate the molarity. N m M where M is the molar mass of this material. Molar mass of Oxygen is 32 gram mole.
 Source: wikihow.com
Source: wikihow.com
More commonly written for this application as. Calculate the mole fraction of HCl and H 2 O in a solution of HCL acid in water containing 20 HCl by weight. Solution The solution contains 20 grams of HCL acid and 80 grams of water. The mole is the SI unit of the measurement for the amount of a substance. The equation to convert moles to mass.
 Source: slideplayer.com
Source: slideplayer.com
Or multiply it by 10 -3 ten to the power -3. An explanation of how to use dimensional analysis to find moles when you know the mass. What i did is with the pH -log H got the value of H where pH ph in the original problem which is 9. Molar mass is generally expressed in gramsmole. Therefore molar mass g mol -1 mass g moles mol.
 Source: syatillakmk.blogspot.com
Source: syatillakmk.blogspot.com
Convert 02 moles of Sodium chloride. While a mole is the unit of measurement for amount of substance in the International System of Units. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. The mole is the SI unit of the measurement for the amount of a substance. N m M where M is the molar mass of this material.
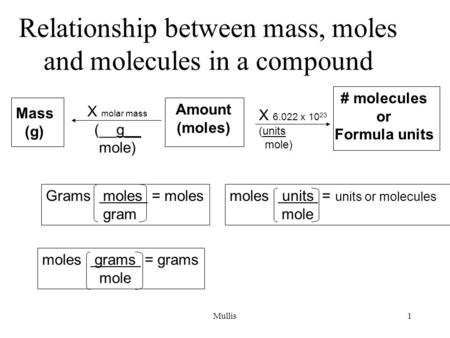 Source: slideplayer.com
Source: slideplayer.com
But if we want to express it in KgMole then devide it by 1000. The formula for moles to grams is given by. Converting grams to moles involves 2 steps. The mole is the SI unit of the measurement for the amount of a substance. More commonly written for this application as.
 Source: youtube.com
Source: youtube.com
By inspection of units we see that dividing the mass in grams by the amount in moles we arrive at a quantity with the units grams per mole g mol-1 which are the units for molar mass. What is the Molality Formula. Where is the molar mass of the substance. Grams Moles x Molar Mass. Molar mass of Oxygen is 32 gram mole.
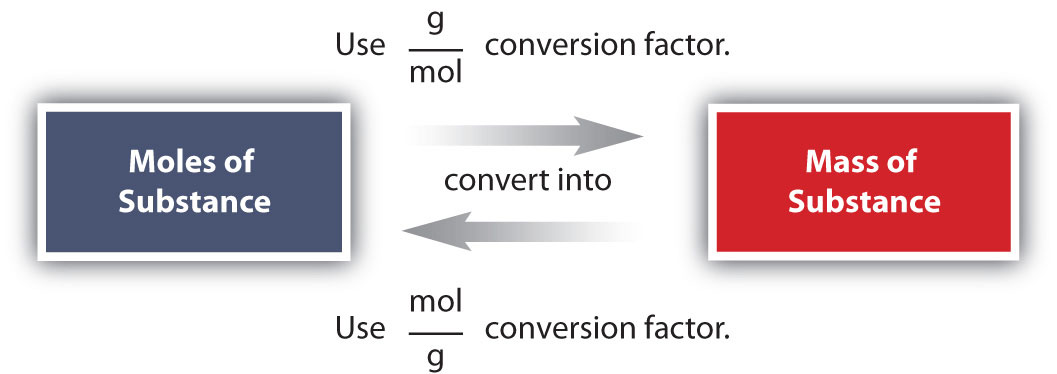 Source: chem.libretexts.org
Source: chem.libretexts.org
N m M where M is the molar mass of this material. And it is 32 103 Kg Mole. Moles of HCl 20365 547 moles of HCl Moles of water 8018 444 moles of H2O. How To Calculate The Quantity Of Moles Multiply wv by. The main equation is.
 Source: wikihow.com
Source: wikihow.com
How To Calculate The Quantity Of Moles Multiply wv by. To convert mass to moles you use the equation n mM n is moles m is mass M Molar mass which is the mass of the atoms in the formula or substance. Find the molar mass of the substance. Ex2 Molar mass of Zn is 655 gram mol and 655 10-3 Kg mol. Convert 02 moles of Sodium chloride.
 Source: slideplayer.com
Source: slideplayer.com
An explanation of how to use dimensional analysis to find moles when you know the mass. While a mole is the unit of measurement for amount of substance in the International System of Units. Also Molar mass of HCl is 365 gramsmole. This chemistry video tutorial explains how to convert the unit grams to moles which is a common conversion step for many stoichiometry questions. Find the number of moles of base to change the ph of buffer solution.
 Source: pinterest.com
Source: pinterest.com
One mole consists of Avogadro number of atoms. Molar mass of Oxygen is 32 gram mole. Molar mass is generally expressed in gramsmole. Divide the number of grams of the compound by its molecular mass. The main equation is.
 Source: youtube.com
Source: youtube.com
This will also help you learning regarding the calculation of half life. To go from moles to grams we need to multiply the number of moles by the molar mass. But wait what actually is a mole. The molar mass is a valuable unit factor for some estimation. And it is 32 103 Kg Mole.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to change mass to moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






