Your How to calculate the number of moles of a compound images are ready. How to calculate the number of moles of a compound are a topic that is being searched for and liked by netizens today. You can Find and Download the How to calculate the number of moles of a compound files here. Find and Download all free photos.
If you’re searching for how to calculate the number of moles of a compound images information linked to the how to calculate the number of moles of a compound keyword, you have come to the ideal site. Our website frequently provides you with suggestions for refferencing the maximum quality video and picture content, please kindly surf and find more enlightening video content and graphics that fit your interests.
How To Calculate The Number Of Moles Of A Compound. 1527 grams Ca 457 gra C and 1828 grams 0. Number of moles 95 8694. X moles x 602 x 1023 SO3 molecules 1mol SO3 Y SO3 molecules. Number of moles Weight of compound in grams molecular weight of compound.
 How To Calculate The Number Of Moles Of An Element Chemistry Youtube From youtube.com
How To Calculate The Number Of Moles Of An Element Chemistry Youtube From youtube.com
For finding out this you have to multiply the mass of solute by its molar mass conversion factor. Number of moles 1092 mol. In other words it tells you the number of. So thats equal to 18016 grams per mole. The answer is the number of. Number of moles formula is.
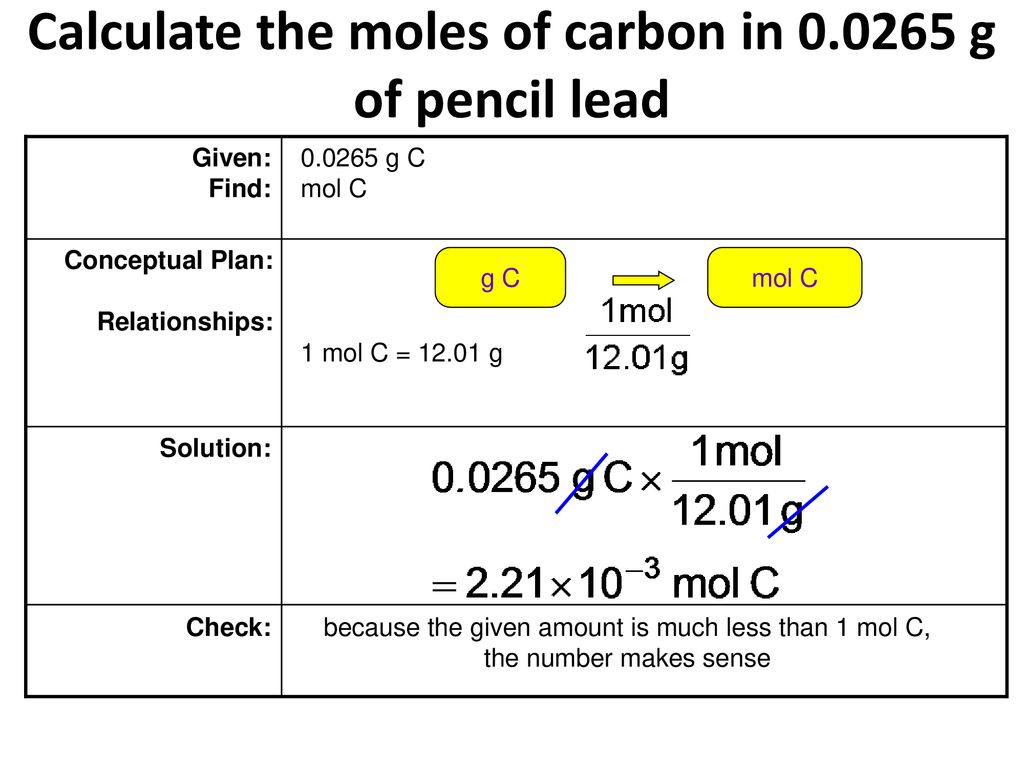
Determine the number of moles in 95g of MnO 2.
Divide the mass of the compound in grams by the molar mass you just calculated. Moles O 545 g O x 1 mol O 1600 g O 341 mol O. To compute for the molar mass two essential parameters are needed and these parameters are mass m and number of moles n. So step one is to convert molecules to moles by dividing by Avogadros number 60221023. Numerically this would be 2 1008 1 1600 18016. Divide the mass of the compound in grams by the molar mass you just calculated.
 Source: slideplayer.com
Source: slideplayer.com
Second step once you have a number of moles is to multiply it by the molar mass of the compound. The number of molecules in a sample is related to moles of compound 1 mole of SO3 will have 602 x 1023 moleculestherefore if you first convert grams t moles then you can convert moles to number of molecules here is the calculation. 48382 g x 1 mol 8006 g X mole SO3. How many atoms of each element are found in. Thus the number of moles in the given sample of.
 Source: thefactfactor.com
Source: thefactfactor.com
Just as with the previous two examples you would use this number 602 1023 Avogadros number to convert from particles atoms or molecules to moles. Now the only confusing thing for me is the 5 moles. The number of grams per mole for each single element is equal to the atomic weight of that element. How to Calculate and solve for the Mass Number of Moles and Molar Mass in Chemistry The Calculator Encyclopedia. Atoms Question 5 Given these masses of elements found in a sample of a compound.
 Source: youtube.com
Source: youtube.com
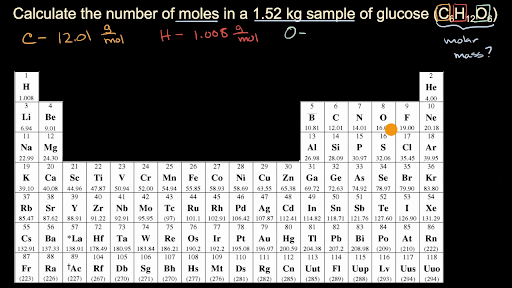
The answer is the number of. In other words it tells you the number of. Number of moles Weight of compound in grams molecular weight of compound. How to Calculate and solve for the Mass Number of Moles and Molar Mass in Chemistry The Calculator Encyclopedia. And we could say grams of glucose C6H12O6 per mole of glucose C6H12O6 and then we can use this 152 kilograms to figure out how many moles we have.
 Source: youtube.com
Source: youtube.com
How many atoms of each element are found in. This is the molar mass of the compound. For example 25 grams of water equals 2518016 or 139 moles. Just as with the previous two examples you would use this number 602 1023 Avogadros number to convert from particles atoms or molecules to moles. Add up the masses of the elements in each compound to find the grams per mole for that compound.
 Source: khanacademy.org
Source: khanacademy.org
Solved Examples On Number Of Moles Formulas. The formula for calculating the molar. You can find this by adding the molar mass of each atom in the formula. If you have 1000 grams. Divide the mass of the compound in grams by the molar mass you just calculated.
 Source: youtube.com
Source: youtube.com
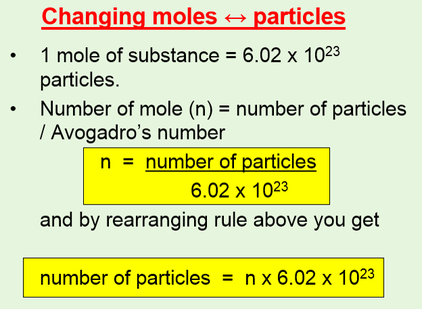
One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. 10 moles H X 2 602 10 23 602 10 23 atoms. Determine the number of moles in 95g of MnO 2. This is the molar mass of the compound. I can only go to the hundredths place for significant figures so 18016.
 Source: chemistryvce.weebly.com
Source: chemistryvce.weebly.com
For finding out this you have to multiply the mass of solute by its molar mass conversion factor. Now the only confusing thing for me is the 5 moles. To find the simplest whole number ratio divide each number by the smallest number of moles. Divide the mass of the compound in grams by the molar mass you just calculated. And we could say grams of glucose C6H12O6 per mole of glucose C6H12O6 and then we can use this 152 kilograms to figure out how many moles we have.
Determine the number of moles in 95g of MnO 2. Number of moles 95 8694. Number of moles Weight of compound in grams molecular weight of compound. How many atoms of each element are found in. To compute for the molar mass two essential parameters are needed and these parameters are mass m and number of moles n.
 Source: surfguppy.com
Source: surfguppy.com
Number of moles Mass of substance Mass of one mole. Moles H 458 g H x 1 mol H 101 g H 453 mol H. So thats equal to 18016 grams per mole. 1527 grams Ca 457 gra C and 1828 grams 0. 09 Calculate the number of atoms of Co in 652 g of Co.
 Source: youtube.com
Source: youtube.com
So step one is to convert molecules to moles by dividing by Avogadros number 60221023. 5 moles H X 2 S O X 4. Then 1000 g 151001 gmol. Whenever you go to the mole divide by Avogadros number. Moles O 545 g O x 1 mol O 1600 g O 341 mol O.
 Source: pharmafactz.com
Source: pharmafactz.com
The formula for calculating the molar. I can only go to the hundredths place for significant figures so 18016. The image above represents the molar mass of a substance. I hope this helped without getting too confusing. If you have 1000 grams.
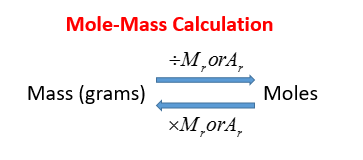 Source: onlinemathlearning.com
Source: onlinemathlearning.com
The number of grams per mole for each single element is equal to the atomic weight of that element. And we could say grams of glucose C6H12O6 per mole of glucose C6H12O6 and then we can use this 152 kilograms to figure out how many moles we have. The numbers of moles of each element are in the same ratio as the number of atoms C H and O in vitamin C. You can find this by adding the molar mass of each atom in the formula. In our example the weight of NaCl is 100 grams and its molecular weight is 5852 gmoles.

Divide the mass of the compound in grams by the molar mass you just calculated. It has units of grams per mole. The answer is the number of moles of that mass of compound. You can find this by adding the molar mass of each atom in the formula. Atoms Question 5 Given these masses of elements found in a sample of a compound.
 Source: wikihow.com
Source: wikihow.com
Divide the mass of the compound in grams by the molar mass you just calculated. Mass of MnO 2 95g. The image above represents the molar mass of a substance. I can only go to the hundredths place for significant figures so 18016. 10 moles H X 2 602 10 23 602 10 23 atoms.
 Source: youtube.com
Source: youtube.com
Number of moles formula is. How many atoms of each element are found in. The answer is the number of moles of that mass of compound. How to Calculate and solve for the Mass Number of Moles and Molar Mass in Chemistry The Calculator Encyclopedia. Just as with the previous two examples you would use this number 602 1023 Avogadros number to convert from particles atoms or molecules to moles.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate the number of moles of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





