Your How to calculate the moles of ions in a compound images are available. How to calculate the moles of ions in a compound are a topic that is being searched for and liked by netizens today. You can Find and Download the How to calculate the moles of ions in a compound files here. Find and Download all royalty-free images.
If you’re searching for how to calculate the moles of ions in a compound images information connected with to the how to calculate the moles of ions in a compound interest, you have pay a visit to the right site. Our site frequently gives you hints for viewing the maximum quality video and image content, please kindly surf and locate more enlightening video content and graphics that fit your interests.
How To Calculate The Moles Of Ions In A Compound. Then using the mass given and molar massive to discover the number of moles the SrF2 present. Calculate the value of Ksp. You need to uncover the molar massive of the whole compound. Lets start with these values.
 Calculate The Mole Fraction Of Total Ions Clutch Prep From clutchprep.com
Calculate The Mole Fraction Of Total Ions Clutch Prep From clutchprep.com
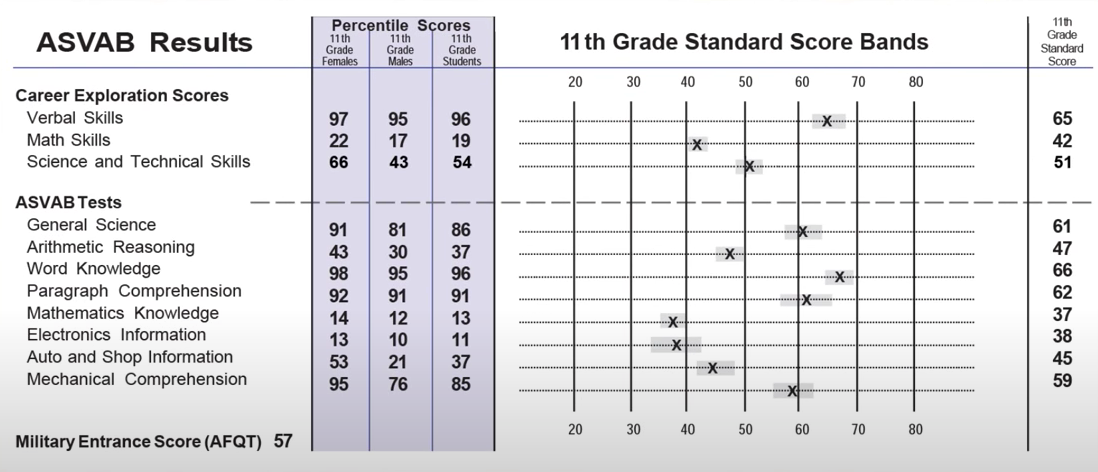
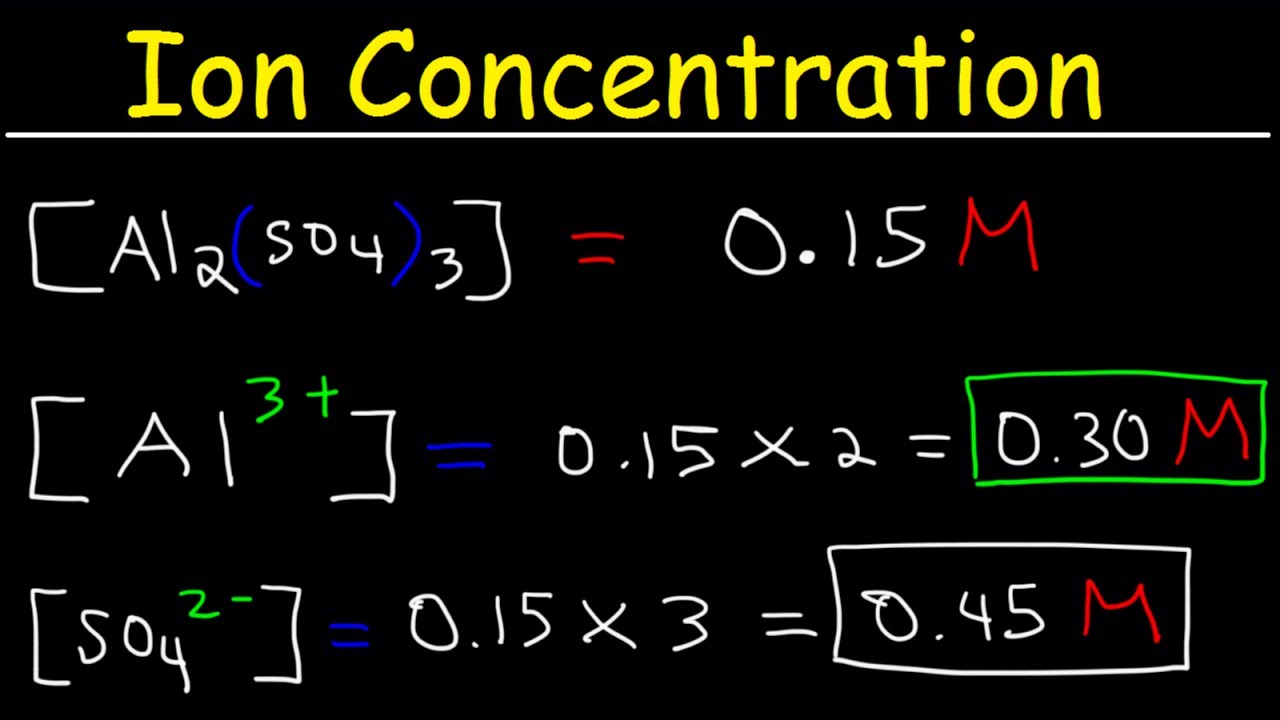
For every mole of MgI2 there is one mole of Mg and 2 moles of I- moles of MgI2 moles of Mg. Moles Molarity x Volume. There room 2 F- ions for every one Sr2 ion so that the all at once charge is neutral. Ions have oxidation numbers equal to their charge. 00035 grams x 1 mole 2781 grams moles of MgI2. Atomic mass of Cl 3545.
You calculate the number of moles by dividing the mass of substance by the substances atomic or molecular weight.
Calculate the value of Ksp. Can you provide an actual question. Calculate the Volume of HCl needed. There room 2 F- ions for every one Sr2 ion so that the all at once charge is neutral. This worked example problem illustrates the steps necessary to calculate the concentration of ions in an aqueous solution in terms of molarityMolarity is one of the most common units of concentration. You need to uncover the molar massive of the whole compound.
 Source: youtube.com
Source: youtube.com
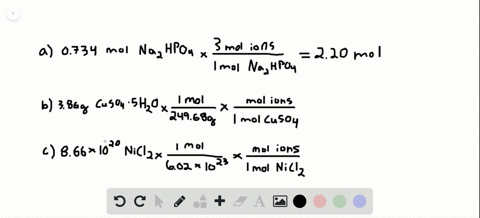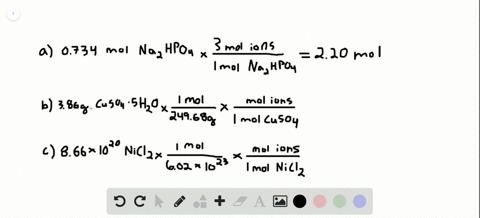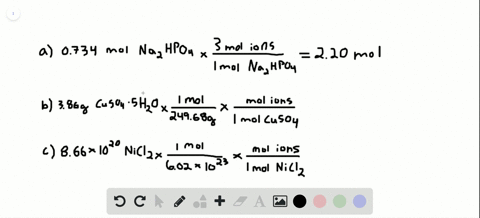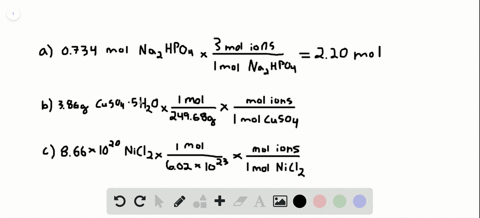
So each mole of K2SO4 produces 3 moles of ions240 moles K2SO4 x 3 moles ionsmole K2SO4 72 moles of ions. Then using the mass given and molar massive to discover the number of moles the SrF2 present. Following the same process outlined above we can determine the molarity of this calcium chloride solution in a few simple steps. 200 mL H 2 O. 10 moles of sodium ions 10 6022 10 23 6022 10 24 sodium ions.
 Source: slideplayer.com
Source: slideplayer.com
Number of mol is calculated by ratio of given mass to the molar mass. How many moles of Cl- ions are there in a solution that is 025M in Cl- and has volume 100 mL Moles 025 moles 10 L 01 L 0025 moles Cl- ions. How to calculate ions in a compound. December 31 2021. You calculate the number of moles by dividing the mass of substance by the substances atomic or molecular weight.
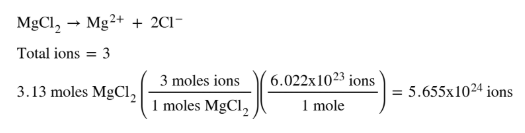 Source: clutchprep.com
Source: clutchprep.com
This worked example problem illustrates the steps necessary to calculate the concentration of ions in an aqueous solution in terms of molarityMolarity is one of the most common units of concentration. 00035 grams x 1 mole 2781 grams moles of MgI2. Calculate the value of Ksp. Molarity moles of solute litres of solution. Find the molarity of the solute.
 Source: youtube.com
Source: youtube.com
00035 grams x 1 mole 2781 grams moles of MgI2. Moles Molarity x Volume. The solubility of Ag2CrO4 in water is 131 x 10-4 molesL. Can you provide an actual question. To find the molarity of the ions first determine the molarity of the solute and the ion-to-solute ratio.
 Source: slideplayer.com
Source: slideplayer.com
There room 2 F- ions for every one Sr2 ion so that the all at once charge is neutral. Number of mol of sodium dfractextgiven masstextmolar mass. Now to calculate the variety of ions present. Find the ionic strength with this online calculator. How to calculate ions in a compound.
 Source: youtube.com
Source: youtube.com
Calculate the number of moles of OH-. The mathematical equation N n N A can be used to find the number of atoms ions or molecules in any amount in moles of atoms ions or molecules. Calculate the value of Ksp. Lets do an example. How to Calculate Number of Moles.
 Source: khanacademy.org
Source: khanacademy.org
More specifically you know that you have. 2K and SO42- ions. Moles Molarity x Volume. Find the molarity of the solute. 200 mL H 2 O.
 Source: youtube.com
Source: youtube.com
This worked example problem illustrates the steps necessary to calculate the concentration of ions in an aqueous solution in terms of molarityMolarity is one of the most common units of concentration. Calculate the Volume of HCl needed. You need to uncover the molar massive of the whole compound. The mathematical equation N n N A can be used to find the number of atoms ions or molecules in any amount in moles of atoms ions or molecules. So each mole of K2SO4 produces 3 moles of ions240 moles K2SO4 x 3 moles ionsmole K2SO4 72 moles of ions.
 Source: youtube.com
Source: youtube.com
How to Calculate Number of Moles. 200 mL H 2 O. Can you provide an actual question. Calculate the Volume of HCl needed. For finding out this you have to multiply the mass of solute by its molar mass conversion factor.
 Source: numerade.com
Source: numerade.com
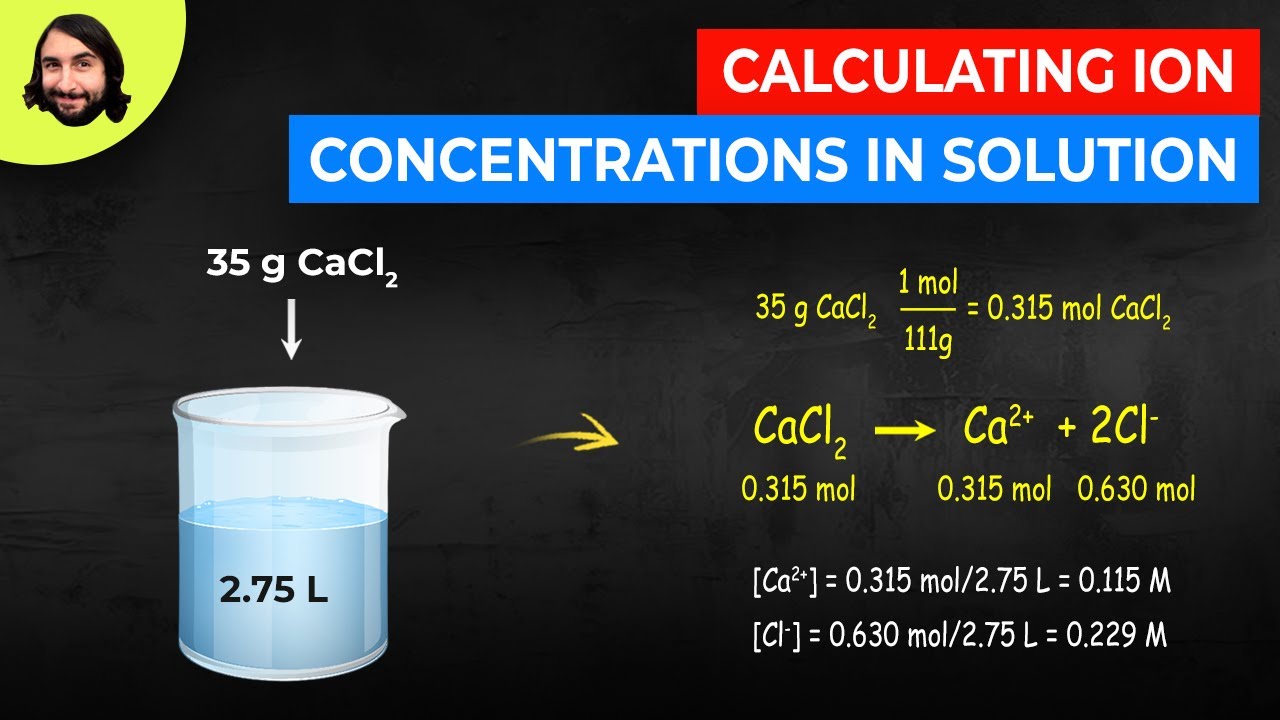
The mathematical equation N n N A can be used to find the number of atoms ions or molecules in any amount in moles of atoms ions or molecules. Calculate the value of Ksp. Atomic mass of Cu 6355. More specifically you know that you have. 100 g CaCl 2.
 Source: youtube.com
Source: youtube.com
Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance. Concentration of each ion using mole ratios record them on top of the equation. How many moles of Cl- ions are there in a solution that is 025M in Cl- and has volume 100 mL Moles 025 moles 10 L 01 L 0025 moles Cl- ions. Then using the mass given and molar massive to discover the number of moles the SrF2 present. I have no idea at all on how to answer.
 Source: clutchprep.com
Source: clutchprep.com
Now to calculate the variety of ions present. 100 g CaCl 2. You calculate the number of moles by dividing the mass of substance by the substances atomic or molecular weight. 5 mol NaOH 3 mol atoms1 mol NaOH 60221023 atoms1 mol atoms 90331024 atoms. This is thoroughly answered here.
 Source: youtube.com
Source: youtube.com
Lets do an example. Find the molarity of the solute. After calculating the number of moles the number of ions will be equal to the product of the number of moles and Avogadros number. December 31 2021. 100 g CaCl 2.
 Source: numerade.com
Source: numerade.com
Calculate the Volume of HCl needed. December 31 2021. 10 moles of sodium ions 10 6022 10 23 6022 10 24 sodium ions. Lets do an example. 10 moles of helium atoms 10 6022 10 23 6022 10 24 helium atoms.

PbCl 2 s – Pb 2 aq 2 Cl - aq K sp Pb 2 Cl - 2. Can you provide an actual question. PbCl 2 s – Pb 2 aq 2 Cl - aq K sp Pb 2 Cl - 2. December 31 2021. 200 mL H 2 O.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate the moles of ions in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.