Your How to calculate the mole ratio of a compound images are available. How to calculate the mole ratio of a compound are a topic that is being searched for and liked by netizens today. You can Find and Download the How to calculate the mole ratio of a compound files here. Download all free vectors.
If you’re looking for how to calculate the mole ratio of a compound pictures information connected with to the how to calculate the mole ratio of a compound keyword, you have visit the right blog. Our site always provides you with suggestions for seeing the maximum quality video and image content, please kindly hunt and find more enlightening video content and images that fit your interests.
How To Calculate The Mole Ratio Of A Compound. The mole ratio can be known as the mole-to. You now have calculated the number of moles of every compound used in this reaction. 2 mol H 2 O. 1191molNa 1 1188molH 1188molH 1 1188molH 1191molC 1 1188molH 3571molO 3 1188molH.
 Molar Ratio Chemistry Youtube From youtube.com
Molar Ratio Chemistry Youtube From youtube.com
First of all before you can use this equation you need to know how many moles of solute are there in the solution. These molar ratios can also be expressed as fractions. O has a constant composition of 5 moles H. The data will be translated into a one significant figure answer. If you want to calculate the mole ratio as a prove you can use the mass weight ratio or atomic ratio. 2 mol O 2.
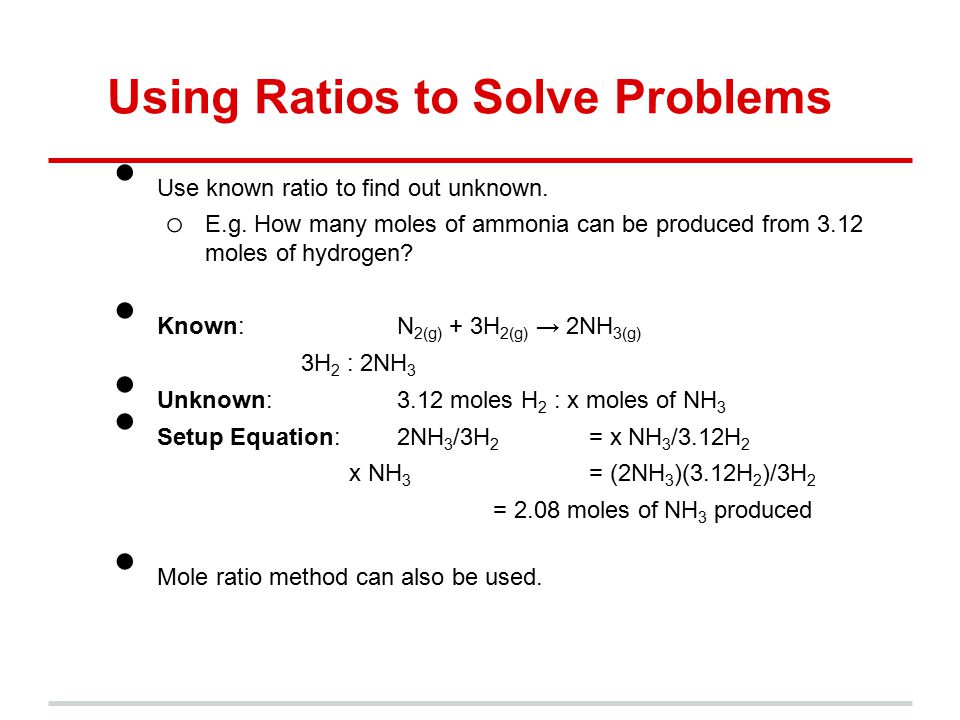
To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant.
120g Al 1 mol Al 2698g Al 0044 48 mol Al. Mass percent mass of element in 1 mole of compound mass of 1 mole of compound x 100. If you want to calculate the mole ratio as a prove you can use the mass weight ratio or atomic ratio. These molar ratios can also be expressed as fractions. The ratio for the amount of oxygen in two moles of nitrogen dioxide is 2-to-2. Then using the same data we will calculate the water of hydration through determining the mole ratio of the decomposition reaction.
 Source: pinterest.com
Source: pinterest.com
Lets say you have a mixture of components 1 and 2 that is 40 mole percent mole fraction 04 component one approximately the blue line labeled liquid on the diagram. 2 mol O 2. 120g Al 1 mol Al 2698g Al 0044 48 mol Al. Calculate the molar ratios. MolAmolB areaANAareaBNB The weight ratio is.
 Source: slideplayer.com
Source: slideplayer.com
Compare the amount of the each element in the compound. The ratio between them is the molar ratio. Well look at several simple ways to find the mole. Calculate the molemole ratio. These molar ratios can also be expressed as fractions.
 Source: youtube.com
Source: youtube.com
First of all before you can use this equation you need to know how many moles of solute are there in the solution. Mass percentage is calculated as the mass of a component divided by the total mass of the mixture multiplied by 100. For each compound find the grams of copper that combine with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. 1 mol CO 2. Convert the mass of the anhydrous salt m2 by dividing it by the molar mass of the anhydrous salt.
 Source: youtube.com
Source: youtube.com
Choose a signal and calculates the area of H separately of each compound. Mass percentage is calculated as the mass of a component divided by the total mass of the mixture multiplied by 100. The smallest number of moles calculated corresponds to hydrogen so divide all of the moles calculated by hydrogens number of moles 1188 to obtain a simplified ratio. For each compound find the grams of copper that combine with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. Convert the mass of each element to moles using the molar mass from the periodic table.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Compare the amount of the each element in the compound. Calculate Moles of Product. The ratio for the amount of oxygen in two moles of nitrogen dioxide is 2-to-2. 2 mol O 2. Convert the mass of each element to moles using the molar mass from the periodic table.
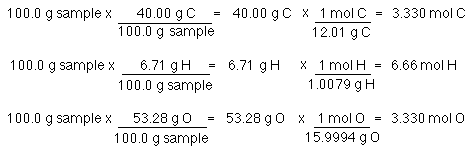 Source: westfield.ma.edu
Source: westfield.ma.edu
To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. How many moles are in a liter. Draw a line vertically from that composition to the bubble point or liquid line. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. Use every parts molar mass to transform the grams of every ingredient to moles.
 Source: quizlet.com
Source: quizlet.com
How many moles are in a liter. Mass percent mass of element in 1 mole of compound mass of 1 mole of compound x 100. For instance mass ratio it is devided by atomic numbers of the element Mr Fe. Draw a line vertically from that composition to the bubble point or liquid line. The mole ratios come from the chemical formula or equation.
 Source: nagwa.com
Source: nagwa.com
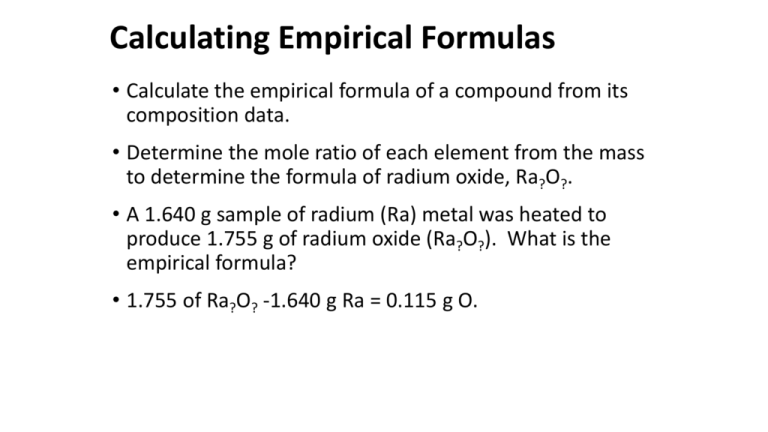
Then draw a horizontal isotherm at that point red line on the diagram above. Then using the same data we will calculate the water of hydration through determining the mole ratio of the decomposition reaction. You now have calculated the number of moles of every compound used in this reaction. In this video youll learn to find the mole ratio from the coefficients in a balanced chemical equation. The mole ratio is the magic that changes from A to B.
 Source: wou.edu
Source: wou.edu
MolAmolB areaANAareaBNB The weight ratio is. The mole ratio is the magic that changes from A to B. For every compound discover the grams of copper that mix with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. Use every parts molar mass to transform the grams of every ingredient to moles. From the coefficients of the equation the mole ratio is 33.
 Source: slideplayer.com
Source: slideplayer.com
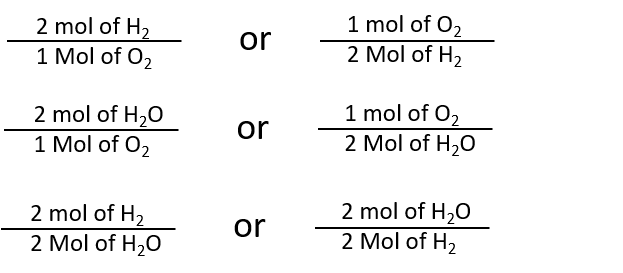
Mole ratios are important because mole ratios allow you change moles of a substance to moles of another substance. These molar ratios can also be expressed as fractions. However this reduces to a 11 ratio. The equality of 1 mole 224 L is the basis for the conversion factor. The mole ratio may be determined by examining the coefficients in front of formulas in a balanced chemical equation.
 Source: youtube.com
Source: youtube.com
The data will be translated into a one significant figure answer. For each one mole of CuSO 4 there are 5 moles of water. Mass percentage is calculated as the mass of a component divided by the total mass of the mixture multiplied by 100. First of all before you can use this equation you need to know how many moles of solute are there in the solution. Lets say you have a mixture of components 1 and 2 that is 40 mole percent mole fraction 04 component one approximately the blue line labeled liquid on the diagram.
 Source: youtube.com
Source: youtube.com
From the coefficients of the equation the mole ratio is 33. 1 mol CH 4. However this reduces to a 11 ratio. Mass percent mass of element in 1 mole of compound mass of 1 mole of compound x 100. For example the ratio for the amount of nitrogen in two moles of nitrogen dioxide 2NO2 is 1-to-2.
 Source: nagwa.com
Source: nagwa.com
Round to the nearest whole number. 1 mol CH 4. The molar ratio is. The mole ratio is the magic that changes from A to B. Common ratios will range from 12 up to 110.
 Source: studylib.net
Source: studylib.net
Then find the ratio of the masses of copper in the two compounds by dividing the larger value by the smaller value. 1 mol CH 4. 1191molNa 1 1188molH 1188molH 1 1188molH 1191molC 1 1188molH 3571molO 3 1188molH. Use every parts molar mass to transform the grams of every ingredient to moles. 1 mol CO 2.
 Source: dummies.com
Source: dummies.com
Draw a line vertically from that composition to the bubble point or liquid line. This will allow you to find the mole ratio. Pure compound are present in a definite constant mass. Then draw a horizontal isotherm at that point red line on the diagram above. O has 2016 grams hydrogen for every 1600 grams oxygen.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate the mole ratio of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






