Your How to calculate the mass of one mole of copper images are available in this site. How to calculate the mass of one mole of copper are a topic that is being searched for and liked by netizens today. You can Get the How to calculate the mass of one mole of copper files here. Find and Download all royalty-free images.
If you’re searching for how to calculate the mass of one mole of copper pictures information related to the how to calculate the mass of one mole of copper interest, you have pay a visit to the ideal site. Our site always provides you with hints for downloading the maximum quality video and image content, please kindly hunt and locate more enlightening video content and graphics that match your interests.
How To Calculate The Mass Of One Mole Of Copper. 1 moles Copper II Fluoride to grams 10154281 grams. From University of Chittagong. Your instructor will tell you if your unknown compound contains more than one mole of copper. 2 moles Copper II Carbonate to grams 2471098 grams.
 Question Video Determining The Mass Of Sodium That Contains The Same Number Of Moles As A Given Number Of Moles Of Copper Nagwa From nagwa.com
Question Video Determining The Mass Of Sodium That Contains The Same Number Of Moles As A Given Number Of Moles Of Copper Nagwa From nagwa.com
Similarly the mass of one molecule of water is 180 amu. Understand molar mass. Of oxygen in CO 2 3244 x 100 727. 2 moles Copper II Fluoride to grams 20308561 grams. At 20C 68F or 29315K at standard atmospheric pressure. Dm 6350590 amu - 6291367 amu 059223 amu Top.
Mass moles molar mass.
Beside above how do you calculate the percentage of copper. Your instructor will tell you if your unknown compound contains more than one mole of copper. Avogadros Number 1 mole 6022 1023particles and the relative atomic mass of copper 1 mole of copper 63546 g are the equalities required to. 3 moles Copper II Carbonate to grams 3706647 grams. Molar mass of Cu 63546 gmol. CopperII Oxide molecular weight.
 Source: pinterest.com
Source: pinterest.com
In this case add up all the atomic masses in the chemical formula and divide by Avogadros number. Of oxygen in CO 2 3244 x 100 727. Thus the molecular mass mCu mN. 3 moles Copper II Fluoride to grams 30462842 grams. 2 moles Copper II Fluoride to grams 20308561 grams.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
TextMolar Mass 107868 for calculations tap Molar Mass Calculator By using moles to grams formula. Molar mass of Cu is 635460 gmol. At 20C 68F or 29315K at standard atmospheric pressure. It is the mass in grams corresponding to the molecular or atomic mass. In this case add up all the atomic masses in the chemical formula and divide by Avogadros number.
 Source: in.pinterest.com
Source: in.pinterest.com
Track your food intake exercise sleep and meditation for free. In this case add up all the atomic masses in the chemical formula and divide by Avogadros number. We can solve this equation to yield the molar mass of an unknown copper compound. 1 10 -6. Similarly the mass of one molecule of water is 180 amu.
 Source: pinterest.com
Source: pinterest.com
Thereforenoof atoms in one mole CuI2 3 60231023 atoms 180691024 atoms. Molar mass of unknown copper compound. 29 protons100728 amuproton 34 neutrons100867 amuneutron or 6350590 amu. Again 1 molecule CuI2 contains 3 atoms. Molar mass of Cu 63546 gmol.
 Source: youtube.com
Source: youtube.com
Step 3 Calculate the percentage. Again 1 molecule CuI2 contains 3 atoms. Step 2 Add the atomic masses of the element required as in the question oxygen 16 16 32. 5 moles Copper II Carbonate to grams 6177745 grams. One mole CuI2 6351272 3175 60231023 molecules.
 Source: chegg.com
Source: chegg.com
Do a quick conversion. One mole CuI2 6351272 3175 60231023 molecules. Convert grams CopperII Oxide to moles or moles CopperII Oxide to grams. Track your food intake exercise sleep and meditation for free. What is the mass in grams of 10 mole of CU.

Simply divide the relative atomic mass of the element by Avogadros number to get the answer in grams. Calculate the mass defect. Convert grams Copper to moles or moles Copper to grams Percent composition by element. Molar mass of Cu is 635460 gmol. It is the mass in grams corresponding to the molecular or atomic mass.
 Source: pinterest.com
Source: pinterest.com
The combined mass is calculated. Step 1 Calculate the molar mass of the compound. Melting Point MP Copper sulfate changes its state from solid to. Step 2 Add the atomic masses of the element required as in the question oxygen 16 16 32. Similarly the mass of one molecule of water is 180 amu.
 Source: brainly.in
Source: brainly.in
Calculate the mass defect. The combined mass is calculated. 1 10 -6. Cu 2 2e- Cus 1 mole of copper is deposited from 2 moles electrons molesCu ½ne- ½ 518 10-5 259 10-5 mol Calculate mass of copper. 1 grams Copper 0015736631731344 mole using the molecular weight calculator and the molar mass of Cu.
 Source: khanacademy.org
Source: khanacademy.org
Molar mass of unknown copper compound. Compound name is copper. Track your food intake exercise sleep and meditation for free. Do a quick conversion. 29 protons100728 amuproton 34 neutrons100867 amuneutron or 6350590 amu.
 Source: slidetodoc.com
Source: slidetodoc.com
Mass moles molar mass. 4 moles Copper II Carbonate to grams 4942196 grams. Melting Point MP Copper sulfate changes its state from solid to. Do a quick conversion. Cu 2 2e- Cus 1 mole of copper is deposited from 2 moles electrons molesCu ½ne- ½ 518 10-5 259 10-5 mol Calculate mass of copper.
 Source: nagwa.com
Source: nagwa.com
Understand molar mass. Copper sulfate weighs 36 gram per cubic centimeter or 3 600 kilogram per cubic meter ie. One mole CuI2 6351272 3175 60231023 molecules. 4 moles Copper II Fluoride to grams 40617123 grams. Copper molecular weight.
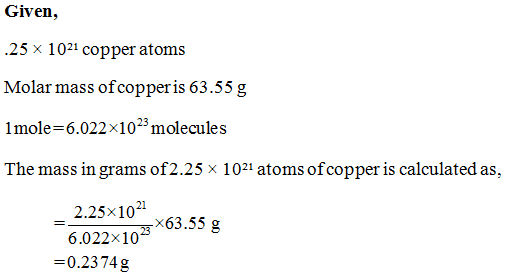 Source: bartleby.com
Source: bartleby.com
Step 2 Add the atomic masses of the element required as in the question oxygen 16 16 32. Step 1 Calculate the molar mass of the compound. Convert grams CopperII Oxide to moles or moles CopperII Oxide to grams. Density of copper is equal to 8 940 kgm³. Thereforenoof atoms in one mole CuI2 3 60231023 atoms 180691024 atoms.
 Source: slideplayer.com
Source: slideplayer.com
4 moles Copper II Fluoride to grams 40617123 grams. Thus the molecular mass mCu mN. 1 10 -6. Answered 1 year ago Author has 28K answers and 8812K answer views. Avogadros Number 1 mole 6022 1023particles and the relative atomic mass of copper 1 mole of copper 63546 g are the equalities required to.
 Source: pinterest.com
Source: pinterest.com
Beside above how do you calculate the percentage of copper. Enter a chemical formula to calculate its molar mass and elemental composition. Density of copper is equal to 8 940 kgm³. Molar mass of Cu. Molar mass of CuO 795454 gmol.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate the mass of one mole of copper by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






