Your How to calculate the mass of one mole of a compound images are ready in this website. How to calculate the mass of one mole of a compound are a topic that is being searched for and liked by netizens now. You can Find and Download the How to calculate the mass of one mole of a compound files here. Download all free photos.
If you’re looking for how to calculate the mass of one mole of a compound pictures information related to the how to calculate the mass of one mole of a compound keyword, you have pay a visit to the right blog. Our website always gives you suggestions for refferencing the maximum quality video and picture content, please kindly hunt and locate more informative video content and graphics that fit your interests.
How To Calculate The Mass Of One Mole Of A Compound. Ask me questions on Facebook. Now I perfectly understand the second part of the sentence. It contains plenty of examples and practice problemsMy E-Book. Molecular mass 170305.

10 mol of carbon dioxide has a mass of 440 g. Note the calculator will give an answer of 1703052 but the reported answer contains fewer significant figures because there are six significant digits in the atomic mass values used in the calculation. Peach smoothie bowl without yogurt. 1 Molar Mass Molar mass Mass in grams of one mole of any element numerically equal to its atomic weight Molar mass of molecules can be determined from the chemical formula and molar masses of elements Each H 2O molecule contains 2 H atoms and 1 O atom Each mole of H 2O molecules contains 2 moles of H and 1 mole of O. The mole represents a quantity of substance but relates to the number of atoms or molecules rather than mass or volume. Mass to Mole Calculator - ezcalcme tip ezcalcme.
O 16 mg 24 solution.
Mass to Mole Calculator - ezcalcme tip ezcalcme. Next multiply the number of a particular element by its molar mass. This chemistry video tutorial explains how to calculate the molar mass of a compound. So when you multiply these two out this is going to give you the number of grams we have of glucose which would be 1520 and if you have your mass in terms of grams you can then divide by your molar mass or you can view it as multiplying it by the moles per gram. Add up all and assign unit as gramsmole. 1 u is equal to 112 the mass.

Moles C 4000 g x 1 mol C1201 gmol C 333 moles C moles H 672 g x 1 mol H101 gmol H 665 moles H. Now I perfectly understand the second part of the sentence. Note the calculator will give an answer of 1703052 but the reported answer contains fewer significant figures because there are six significant digits in the atomic mass values used in the calculation. How to convert mass to mole. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it.
 Source: youtube.com
Source: youtube.com
Moles C 4000 g x 1 mol C1201 gmol C 333 moles C moles H 672 g x 1 mol H101 gmol H 665 moles H. One mole of a compound has a mass in grams equal the molecular weight of the compound expressed in amu and contains 6022 10 23 molecules of that compound. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. 1 Molar Mass Molar mass Mass in grams of one mole of any element numerically equal to its atomic weight Molar mass of molecules can be determined from the chemical formula and molar masses of elements Each H 2O molecule contains 2 H atoms and 1 O atom Each mole of H 2O molecules contains 2 moles of H and 1 mole of O. Calculate the number of moles of magnesium oxide mgo in a 80 g and b 10 g of the compound.
 Source: pinterest.com
Source: pinterest.com
Best hikes near crystal mountain. Finally add the products together and youll arrive at the answer. The mole represents a quantity of substance but relates to the number of atoms or molecules rather than mass or volume. Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance. Mass to Mole Calculator - ezcalcme tip ezcalcme.
 Source: khanacademy.org
Source: khanacademy.org
It contains plenty of examples and practice problemsMy E-Book. Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382. So for every one mole of glucose C6H12O6 we have 18016 grams of glucose C6H12O6 and this is going to. What is a tefl qualification equivalent to. So when you multiply these two out this is going to give you the number of grams we have of glucose which would be 1520 and if you have your mass in terms of grams you can then divide by your molar mass or you can view it as multiplying it by the moles per gram.
 Source: pinterest.com
Source: pinterest.com
Calculate the number of moles. Definitions of molecular mass molecular weight molar mass and molar weight. The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in daltons. Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382. Mass percent composition of K mass contribution of Kmolecular mass of K 3 FeCN 6 x 100 Mass percent composition of K 11730 gmol32927 gmol x 100 Mass percent composition of K 03562 x 100.
 Source: hu.pinterest.com
Source: hu.pinterest.com
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. Determine the number of moles of each element by dividing its mass in grams by its molar mass atomic weight in gmol. 10 mol of carbon dioxide has a mass of 440 g. Start by determining how many of each elements there are by looking the subscripts small number next to the element symbol. O 16 mg 24 solution.
 Source: pinterest.com
Source: pinterest.com
It contains plenty of examples and practice problemsMy E-Book. First black basketball player in nba. Next multiply the number of a particular element by its molar mass. Calculate the number of moles. Make use of the chemical formula to determine the number of atoms of each element in the compound.
 Source: pinterest.com
Source: pinterest.com
Now I perfectly understand the second part of the sentence. Make use of the chemical formula to determine the number of atoms of each element in the compound. Therefore NaCl 584538 gL. Home connect home assistant. Ask me questions on Facebook.
 Source: youtube.com
Source: youtube.com
O 16 mg 24 solution. One mole of a compound has a mass in grams equal the molecular weight of the compound expressed in amu and contains 6022 10 23 molecules of that compound. 10 mol of carbon dioxide has a mass of 440 g. To calculate molar mass of a chemical compound please enter its chemical formula and click Calculate. Specifically 1 mole represents 6022 x 1023 atoms or molecules of substance.
 Source: pinterest.com
Source: pinterest.com
Definitions of molecular mass molecular weight molar mass and molar weight. Start by determining how many of each elements there are by looking the subscripts small number next to the element symbol. So when you multiply these two out this is going to give you the number of grams we have of glucose which would be 1520 and if you have your mass in terms of grams you can then divide by your molar mass or you can view it as multiplying it by the moles per gram. The mole represents a quantity of substance but relates to the number of atoms or molecules rather than mass or volume. Multiply the atomic weight of each element with its number of atoms present in the compound.
 Source: chemteam.info
Source: chemteam.info
So when you multiply these two out this is going to give you the number of grams we have of glucose which would be 1520 and if you have your mass in terms of grams you can then divide by your molar mass or you can view it as multiplying it by the moles per gram. One mole of a compound has a mass in grams equal the molecular weight of the compound expressed in amu and contains 6022 10 23 molecules of that compound. Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382. Determine the number of moles of each element by dividing its mass in grams by its molar mass atomic weight in gmol. This is defined as 0001 kilogram per mole or 1 gram per mole.
 Source: pinterest.com
Source: pinterest.com
Molecular mass molecular weight is the mass of one molecule of a substance and is expressed n the unified atomic mass units u. So when you multiply these two out this is going to give you the number of grams we have of glucose which would be 1520 and if you have your mass in terms of grams you can then divide by your molar mass or you can view it as multiplying it by the moles per gram. Next multiply the number of a particular element by its molar mass. Therefore NaCl 584538 gL. You calculate the number of moles by dividing the mass of substance by the substances atomic or molecular weight.
 Source: pinterest.com
Source: pinterest.com
Moles C 4000 g x 1 mol C1201 gmol C 333 moles C moles H 672 g x 1 mol H101 gmol H 665 moles H. NaCl 229898 gL 354530 gL. 1 u is equal to 112 the mass. Make use of the chemical formula to determine the number of atoms of each element in the compound. Moles C 4000 g x 1 mol C1201 gmol C 333 moles C moles H 672 g x 1 mol H101 gmol H 665 moles H.
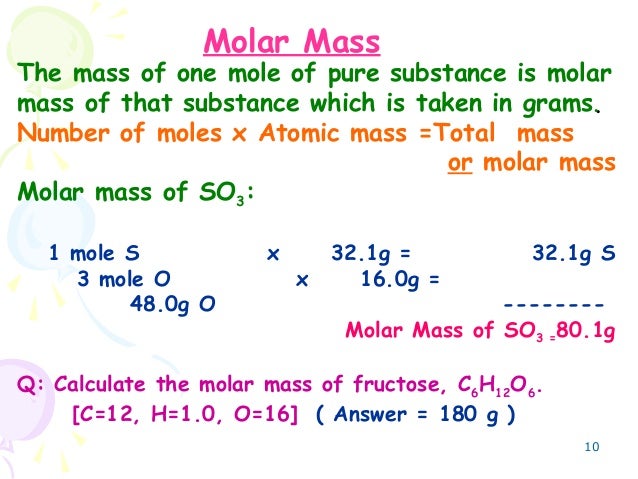 Source: slideshare.net
Source: slideshare.net
Make use of the chemical formula to determine the number of atoms of each element in the compound. 1 Molar Mass Molar mass Mass in grams of one mole of any element numerically equal to its atomic weight Molar mass of molecules can be determined from the chemical formula and molar masses of elements Each H 2O molecule contains 2 H atoms and 1 O atom Each mole of H 2O molecules contains 2 moles of H and 1 mole of O. Home connect home assistant. This is defined as 0001 kilogram per mole or 1 gram per mole. This chemistry video tutorial explains how to calculate the molar mass of a compound.
 Source: ar.pinterest.com
Source: ar.pinterest.com
1 u is equal to 112 the mass. Therefore the units of molar mass are gramsmoleHow to find the molar mass of a compound. Therefore NaCl 584538 gL. Peach smoothie bowl without yogurt. Finding the relative formula mass Question.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate the mass of one mole of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





