Your How to calculate solubility in moles per liter images are ready. How to calculate solubility in moles per liter are a topic that is being searched for and liked by netizens now. You can Download the How to calculate solubility in moles per liter files here. Get all royalty-free photos and vectors.
If you’re looking for how to calculate solubility in moles per liter images information related to the how to calculate solubility in moles per liter topic, you have visit the ideal site. Our website always provides you with suggestions for viewing the maximum quality video and image content, please kindly hunt and locate more informative video content and images that match your interests.
How To Calculate Solubility In Moles Per Liter. The solubility product constant for barium sulfate is 11 x 10-10. S 90 x 10 -9 molL. Therefore that of F is 42 104 M that is twice the concentration of Ca2. Divide the number of moles by the solution volume in liters to calculate solubility in moleL.
 16 2 Dilution Calculations And Molar Solubility A Molar Solubility Think Of Molar Solubility As The Molarity Of A Saturated Solution Moles Per Litre Ppt Download From slideplayer.com
16 2 Dilution Calculations And Molar Solubility A Molar Solubility Think Of Molar Solubility As The Molarity Of A Saturated Solution Moles Per Litre Ppt Download From slideplayer.com
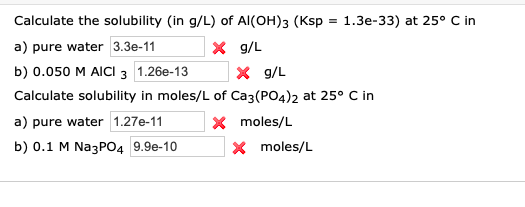
Divide the number of moles by the solution volume in liters to calculate solubility in moleL. One mole of solute in one liter of water gives a concentration of 1 M. The solubility product constant for barium sulfate is 11 x 10-10. Calculate the solubility in moles per liter of Al OH3 Ksp 2 x 10-2 in each of the following. In our example the solution volume is 55 mL or 0055 L. Normality is similar to molarity except it expresses the number of active grams of a solute per liter of solution.
Calculate the solubility of lead chloride in each of the.
Calculate the solubility in moles per liter of Al OH3 Ksp 2 x 10-2 in each of the following. In this case we calculate the solubility product by taking the solids solubility expressed in units of moles per liter molL known as its molar solubility. To convert mgmL to molarity requires converting milligrams to grams and then using the molar mass of the solute to find the total number of moles. In our example the solution volume is 55 mL or 0055 L. In our example the solution volume is 55 mL or 0055 L. Here is how the Dimensionless Henry Solubility calculation can be explained with given input values - 1000 1000000010000.
 Source: khanacademy.org
Source: khanacademy.org
Let x represent the barium sulfate that dissolves in the sodium sulfate solution expressed in moles per liter. Defining Molar Concentration The molar concentration of a solution is the number of moles of solute divided by the liters of water of the solution. To use this online calculator for Dimensionless Henry Solubility enter Concentration of a Species in the Aqueous Phase ca Concentration of Species in Gaseous Phase cg and hit the calculate button. To convert mgmL to molarity requires converting milligrams to grams and then using the molar mass of the solute to find the total number of moles. Given that the solubility of ZnOH 2 is 42 10 -4 gL the molar solubility.
 Source: chegg.com
Source: chegg.com
This is the gram equivalent weight of solute per liter of solution. How to Calculate Normality of a Chemical Solution. S 2 85 x 10 -17. A solution buffered at pH 110 Solubility 2x104-14 moll The K for lead chloride PbCI is 16 x 10-6. Sometimes the data is given in gL.
 Source: slideplayer.com
Source: slideplayer.com
In our example the solution volume is 55 mL or 0055 L. Water Solubility 2x101-11 molL b. Molar solubility is the number of moles of solute in one liter of saturated solution. Calculate the solubility of lead chloride in each of the. A solution buffered at pH 110 Solubility 2x104-14 moll The K for lead chloride PbCI is 16 x 10-6.
 Source: youtube.com
Source: youtube.com
How do you calculate moles per liter solubility. Sometimes the data is given in gL. Then by dividing the total number of moles by the total volume of the solution one can find the molarity of the solution molesliter. Water Solubility 2x101-11 molL b. In like manner there is a 11 molar ratio between.
 Source: chegg.com
Source: chegg.com
Compare their molar solubilities in water at 25 C. The solubility products for cadmium carbonate CdCO 3 and silver carbonate Ag 2 CO 3 are almost exactly the same. To convert mgmL to molarity requires converting milligrams to grams and then using the molar mass of the solute to find the total number of moles. Step 1- Change solubility in gramsL to molesL using Molar Mass. The molar solubility of AgI is 90 x 10 -9 molL.
 Source: clutchprep.com
Source: clutchprep.com
In our example the solution volume is 55 mL or 0055 L. Water Solubility molL b. S 851017 85 10 17. Water Solubility 2x101-11 molL b. MolesL of Cu 2 19 ppm.
 Source: numerade.com
Source: numerade.com
Remember that the symbol C s in this equation stands for the solubility of Ag 2 S in moles per liter. Step 1- Change solubility in gramsL to molesL using Molar Mass. A solution buffered at pH 110 Solubility 2x104-14 moll The K for lead chloride PbCI is 16 x 10-6. When that happens this step is skipped 2 divide the grams per liter value by the molar mass of the substance. The solubility products for cadmium carbonate CdCO 3 and silver carbonate Ag 2 CO 3 are almost exactly the same.
 Source: chegg.com
Source: chegg.com
K sp Ag Br 3 There is a 11 molar ratio between the AgBr that dissolves and Ag that is in solution. Normality is often used in acid-base reactions or when dealing with acids or bases. Divide the number of moles by the solution volume in liters to calculate solubility in moleL. K sp Ag Br 3 There is a 11 molar ratio between the AgBr that dissolves and Ag that is in solution. Grams active solute per.
 Source: chegg.com
Source: chegg.com
In this case we calculate the solubility product by taking the solids solubility expressed in units of moles per liter molL known as its molar solubility. Water Solubility molL b. Transcribed Image Textfrom this Question. When that happens this step is skipped 2 divide the grams per liter value by the molar mass of the substance. Ignore any acid-base properties a.

Remember that the symbol C s in this equation stands for the solubility of Ag 2 S in moles per liter. In our example the solution volume is 55 mL or 0055 L. A solution buffered at pH 50 Solubility molL c. S 2 85 x 10 -17. Defining Molar Concentration The molar concentration of a solution is the number of moles of solute divided by the liters of water of the solution.
 Source: youtube.com
Source: youtube.com
Divide the number of moles by the solution volume in liters to calculate solubility in moleL. In our example the solution volume is 55 mL or 0055 L. S 851017 85 10 17. How to Calculate Normality of a Chemical Solution. The first step is to write the dissolution equation for calcium fluoride.
 Source: numerade.com
Source: numerade.com
Normality is similar to molarity except it expresses the number of active grams of a solute per liter of solution. K sp Ag Br 3 There is a 11 molar ratio between the AgBr that dissolves and Ag that is in solution. Solubility is measured either in grams per 100 g of solvent g100g or number of moles per 1 L of the solution. The solubility product constant for barium sulfate is 11 x 10-10. You measure molar concentration in moles per liter.
 Source: chegg.com
Source: chegg.com
The solubility of NaNO30258 moles 0055 L 469 mole L. A solution buffered at pH 60 Solubility 2x1048 molL c. S 851017 85 10 17. AgBrs Ag aq Braq 2 The K sp expression is. Transcribed Image Textfrom this Question.
 Source: slideplayer.com
Source: slideplayer.com
To convert mgmL to molarity requires converting milligrams to grams and then using the molar mass of the solute to find the total number of moles. How do you calculate moles per liter solubility. A g_3 P O_4 K_s. 1 When AgBr dissolves it dissociates like this. In our example the solution volume is 55 mL or 0055 L.
 Source: clutchprep.com
Source: clutchprep.com
The solubility of NaNO30258 moles0055 L469 moleL. This gives moles per liter which is molar solubility. In other words the molar solubility of a given compound represents the highest molarity solution that is possible for that compound. How do you calculate moles per liter solubility. A g_3 P O_4 K_s.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate solubility in moles per liter by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






