Your How to calculate moles of ions images are ready in this website. How to calculate moles of ions are a topic that is being searched for and liked by netizens now. You can Find and Download the How to calculate moles of ions files here. Get all royalty-free photos.
If you’re searching for how to calculate moles of ions pictures information linked to the how to calculate moles of ions topic, you have come to the right site. Our site frequently gives you hints for refferencing the highest quality video and picture content, please kindly hunt and locate more enlightening video content and graphics that match your interests.
How To Calculate Moles Of Ions. Moles Molarity x Volume. So whatever the molar concentration of the solution is the concentration of sodium ions will be twice the concentration of sulfate ions which will be the same as the solution concentration. So 027 moles will contain. In a balanced neutralization equation the moles of H ions supplied by the acid will be equal to the moles of OH ions supplied by the base.
 Calculating Ion Concentrations In Solution Youtube From youtube.com
Calculating Ion Concentrations In Solution Youtube From youtube.com
4 e- 4 H 2 Ol 2 H 2 g 4 OH-aq Calculate the number of moles of electrons. 2K and SO42- ions. Two moles of HCl are required to completely neutralize one mole of BaOH 2. Following the same process outlined above we can determine the molarity of this calcium chloride solution in a few simple steps. Find the ion-to-solute ratio. The beginning weight tells how much copper you started with.
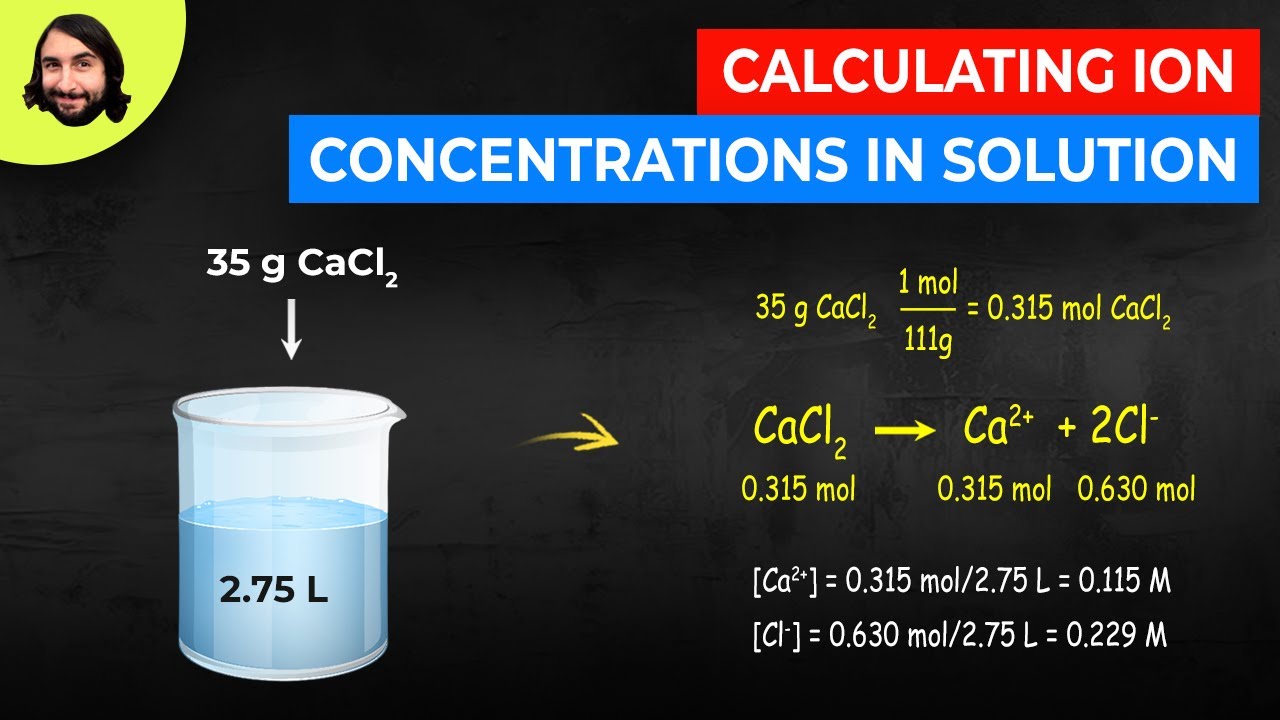
This worked example problem illustrates the steps necessary to calculate the concentration of ions in an aqueous solution in terms of molarityMolarity is one of the most common units of concentration.
Calculate the Volume of HCl needed. Each mole of Co2Cl-2 contains 1 mole of Ca2 ions and 2 moles of Cl- ions making 3 moles of ions in total. Concentration of each ion using mole ratios record them on top of the equation. Hydrogen is produced during the reduction of water at the cathode. Divide the mass of copper that reacted by its atomic. Lets start with these values.
 Source: youtube.com
Source: youtube.com
1 mole 6022 times 1023 particle or atoms or molecules or electrons or protons etc. Find the ionic strength with this online calculator. Once we calculate the moles of iodine titrated the relationship between moles of iodide and iodine would be. Number of ions left textnumber of moles righttextX left N_A right where N_A Avogadros number. This worked example problem illustrates the steps necessary to calculate the concentration of ions in an aqueous solution in terms of molarityMolarity is one of the most common units of concentration.
 Source: khanacademy.org
Source: khanacademy.org
IO3- 5I- 6H — 3I 2 3H 2 O. Once we calculate the moles of iodine titrated the relationship between moles of iodide and iodine would be. Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place. As the pOH increases the concentration of hydroxide ions in the solution decreases. OH - 10 -pOH.
 Source: youtube.com
Source: youtube.com
More specifically you know that you have. CuCl 2 dissociates by the reaction CuCl 2 Cu 2 2Cl - Ionsolute Number of. POH -log 10 OH - OH - is the concentration of hydroxide ions in mol L -1 molL or M OH - in mol L -1 can be calculated using the equation formula. Calculate the number of hydrogen ions. The equation for the formation of I2 from I- ions is this.
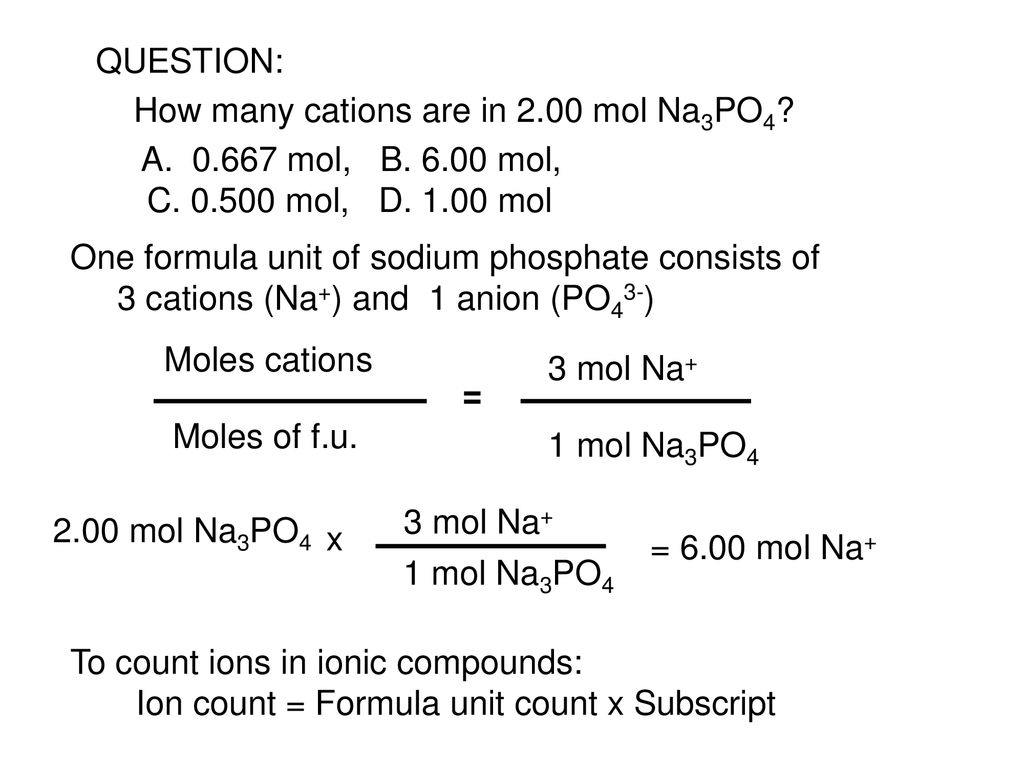 Source: slideplayer.com
Source: slideplayer.com
POH -log 10 OH - OH - is the concentration of hydroxide ions in mol L -1 molL or M OH - in mol L -1 can be calculated using the equation formula. A cm 3 is a mL and a dm 3 is a liter. Calculate the mass of copper that reacted from the two weights that you have. Hydrogen is produced during the reduction of water at the cathode. Lets start with these values.
 Source: youtube.com
Source: youtube.com
Lets do an example. 2K and SO42- ions. A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons. In a balanced neutralization equation the moles of H ions supplied by the acid will be equal to the moles of OH ions supplied by the base. So the correct answer is a.
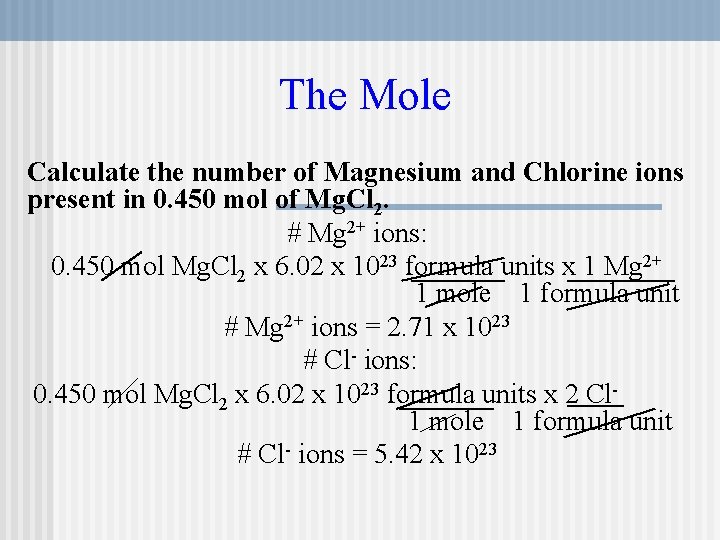 Source: slidetodoc.com
Source: slidetodoc.com
Moles Molarity x Volume. 10 moles of helium atoms 10 6022 10 23 6022 10 24 helium atoms. 100 g CaCl 2. NaClaq Na aq Cl aq So if every mole of sodium chloride produces One mole of sodium cations it follows that the number of moles of sodium cation present in your solution will be equal to the number of moles of sodium chloride you dissolved to create this solution. Use the value for the number of H to calculate the pH and compare this value to the value given in the question.
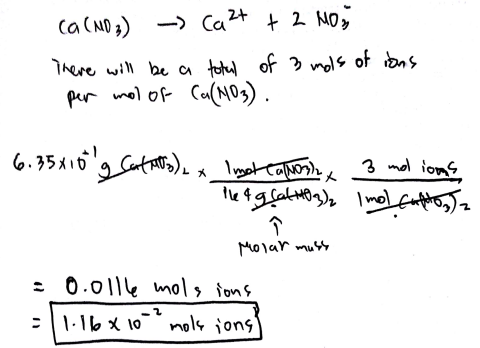 Source: clutchprep.com
Source: clutchprep.com
A cm 3 is a mL and a dm 3 is a liter. Calculate the Volume of HCl needed. Calculate the number of moles of OH-. Find the ion molarity. Formula to calculate moles.
 Source: slideplayer.com
Source: slideplayer.com
So the correct answer is a. The beginning weight tells how much copper you started with. The equation for this half-reaction is. So the correct answer is a. Concentration of each ion using mole ratios record them on top of the equation.
 Source: youtube.com
Source: youtube.com
How do you convert from moles to ions. NaClaq Na aq Cl aq So if every mole of sodium chloride produces One mole of sodium cations it follows that the number of moles of sodium cation present in your solution will be equal to the number of moles of sodium chloride you dissolved to create this solution. Formula to calculate moles. Use the value for the number of H to calculate the pH and compare this value to the value given in the question. 10 moles of sodium ions 10 6022 10 23 6022 10 24 sodium.
 Source: numerade.com
Source: numerade.com
Concentration of each ion using mole ratios record them on top of the equation. Concentration of each ion using mole ratios record them on top of the equation. The equation nNNA can be used to calculate. Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place. 10 moles of sodium ions 10 6022 10 23 6022 10 24 sodium.
 Source: slideplayer.com
Source: slideplayer.com
The solubility of Ag2CrO4 in water is 131 x 10-4 molesL. 1 mole Avogadros Number of particles 602 10 23 particles 10-7 mol H 10-7 602 10 23 60 10 16 hydrogen ions Is your answer plausible. Number of ions left textnumber of moles righttextX left N_A right where N_A Avogadros number. 10 moles of sodium ions 10 6022 10 23 6022 10 24 sodium. Lets do an example.
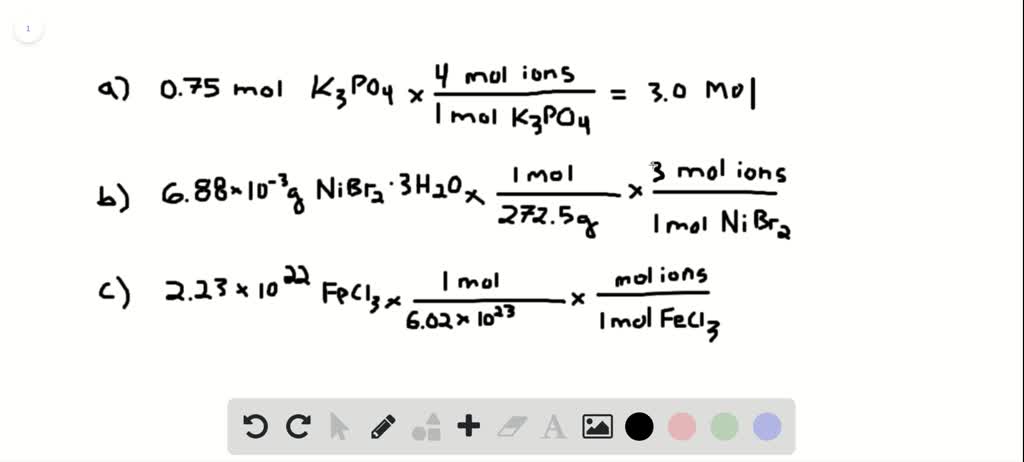 Source: numerade.com
Source: numerade.com
Calculate moles of H. Lets do an example. Moles of iodide 53 moles of iodine. Find the ion-to-solute ratio. How to Calculate Number of Moles.
 Source: slideplayer.com
Source: slideplayer.com
POH -log 10 OH - OH - is the concentration of hydroxide ions in mol L -1 molL or M OH - in mol L -1 can be calculated using the equation formula. Ions have oxidation numbers equal to their charge. Calculate the value of Ksp. So the correct answer is a. Hydrogen is produced during the reduction of water at the cathode.
 Source: youtube.com
Source: youtube.com
So whatever the molar concentration of the solution is the concentration of sodium ions will be twice the concentration of sulfate ions which will be the same as the solution concentration. How to find moles of ions. Remember at STP 1 mole of any gas occupies 224 L Write the equation for the half-reaction that takes place. So each mole of K2SO4 produces 3 moles of ions240 moles K2SO4 x 3 moles ionsmole K2SO4 72 moles of ions. Find the ion-to-solute ratio.
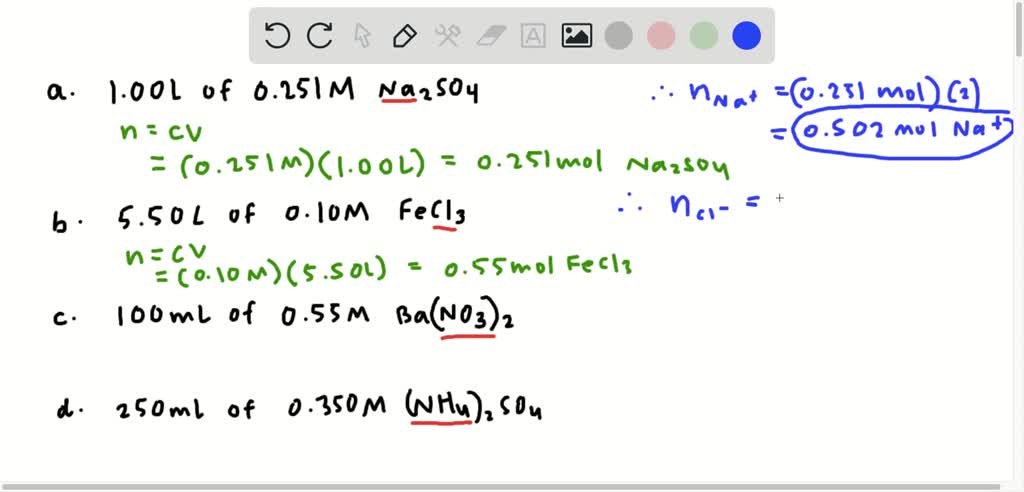 Source: numerade.com
Source: numerade.com
Moles Molarity x Volume. Lets start with these values. Moles of atoms n if you know the number of atoms present N moles of ions n if you know the number of ions present N. Find the molarity of the solute. Number of ions left textnumber of moles righttextX left N_A right where N_A Avogadros number.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate moles of ions by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






