Your How to calculate moles of compound images are available. How to calculate moles of compound are a topic that is being searched for and liked by netizens today. You can Get the How to calculate moles of compound files here. Download all free photos and vectors.
If you’re searching for how to calculate moles of compound pictures information connected with to the how to calculate moles of compound keyword, you have come to the ideal site. Our website always gives you hints for downloading the maximum quality video and image content, please kindly search and find more informative video articles and graphics that fit your interests.
How To Calculate Moles Of Compound. What number of moles Iron in 1 grams. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. Now to calculate its molar mass we add up all of the molar masses of each atom. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms.
 Gcse Science Revision Chemistry Calculating Moles Of A Compound Youtube From youtube.com
Gcse Science Revision Chemistry Calculating Moles Of A Compound Youtube From youtube.com
100 g CaCl 2. 200 mL H 2 O. For example one molecule of H2O has two atoms of Hydrogen H. NaCl 229898 gL 354530 gL. Ions have oxidation numbers equal to their charge. From the equation 2 mol of.
Lets start with these values.
Following the same process outlined above we can determine the molarity of this calcium chloride solution in a few simple steps. An elements molar mass in gmol is numerically equal to the elements atomic mass in amu. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. If you know a substance contains 3011 10 23 particles of the substance then the moles of substance will be 3011 10 23 6022 10 23 05 mol. Number of moles of NaOH mass relative formula mass 20 40 05 mol. In other words it tells you the number of.
 Source: pharmafactz.com
Source: pharmafactz.com
It has units of grams per mole. This is the molar mass of the compound. Find the ionic strength with this online calculator. Next we will convert the given mass to moles of solute present in the solution. If you had 350 L of CO at STP then you would have 350 L 1 mol224 L 156 mol of CO Lastly it has been calculated that one mole of any substance contains 602 1023 atoms if you are talking about pure elements molecules if you are talking about covalent compounds or the general term particles used primarily for inorganic compounds.
 Source: surfguppy.com
Source: surfguppy.com
This is the molar mass of the compound. If you had 350 L of CO at STP then you would have 350 L 1 mol224 L 156 mol of CO Lastly it has been calculated that one mole of any substance contains 602 1023 atoms if you are talking about pure elements molecules if you are talking about covalent compounds or the general term particles used primarily for inorganic compounds. 25000 g of Cl2 are used and there are 70506 gmol of Cl2. In other words it tells you the number of. Therefore NaCl 584538 gL.
 Source: dummies.com
Source: dummies.com
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. And we could say grams of glucose C6H12O6 per mole of glucose C6H12O6 and then we can use this 152 kilograms to figure out how many moles we have. How to Calculate Number of Moles. If you had 350 L of CO at STP then you would have 350 L 1 mol224 L 156 mol of CO Lastly it has been calculated that one mole of any substance contains 602 1023 atoms if you are talking about pure elements molecules if you are talking about covalent compounds or the general term particles used primarily for inorganic compounds. It contains plenty of examples and practice problemsMy E-Book.
 Source: youtube.com
Source: youtube.com
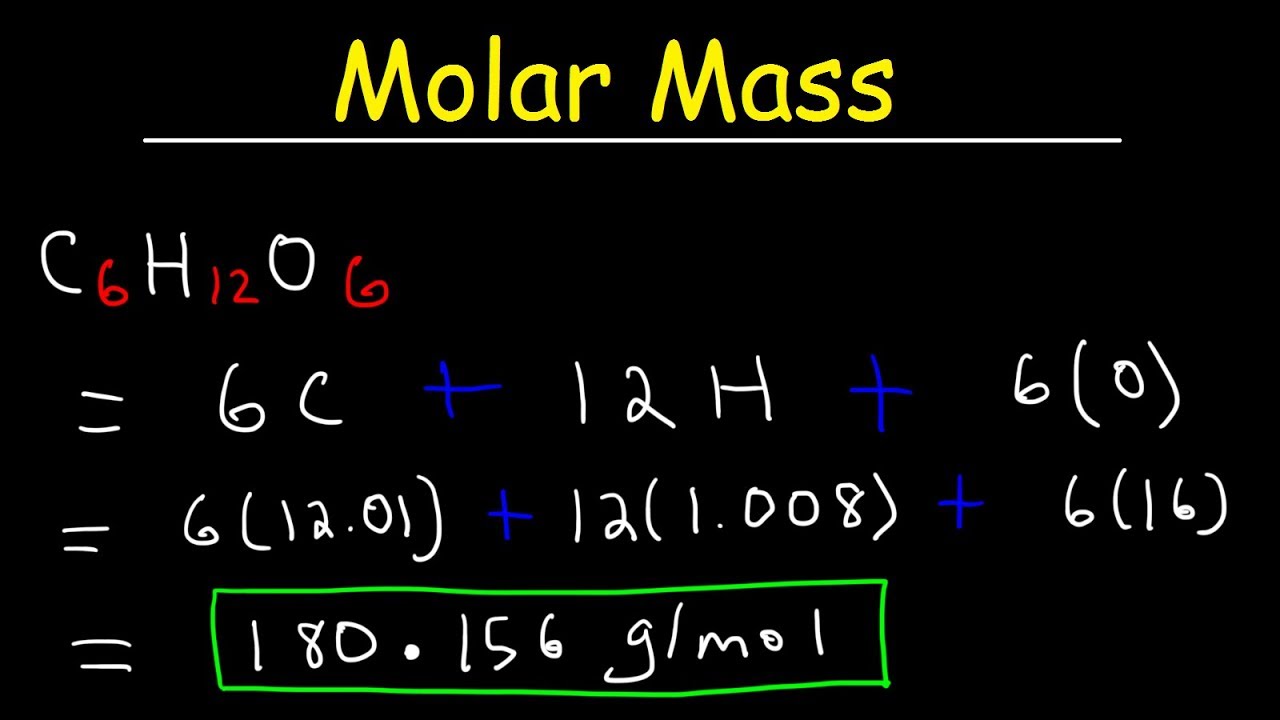
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. NaCl Na Cl. So thats equal to 18016 grams per mole. From compound to compound to a number of atoms in a molecule vary. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom.
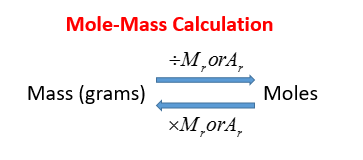 Source: onlinemathlearning.com
Source: onlinemathlearning.com
An elements molar mass in gmol is numerically equal to the elements atomic mass in amu. Next we will convert the given mass to moles of solute present in the solution. M r of NaOH 23 16 1 40. Use the molar mass calculator to easily get an accurate set of data describing any compound. If you know a substance contains 3011 10 23 particles of the substance then the moles of substance will be 3011 10 23 6022 10 23 05 mol.
 Source: youtube.com
Source: youtube.com
NaCl 229898 gL 354530 gL. 200 mL H 2 O. We assume you are. Glucose molecular formula is c 6 h 12 o 6. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms.
 Source: khanacademy.org
Source: khanacademy.org
If you know a substance contains 3011 10 23 particles of the substance then the moles of substance will be 3011 10 23 6022 10 23 05 mol. Fourth convert that to mols. M 9 12 g m o l 1 1 13 g m o l 1 14 g m o l 1 3 16 g m o l 1 183 g m o l 1. So 7 g has moles 7 g X 1 mol 9521 gmol 00735 moles. Determining Number of Moles of a Compound With Known Mass Once youve found the molecular weight you know the weight of one mole of a compound.
 Source: studylib.net
Source: studylib.net
This chemistry video tutorial explains how to calculate the molar mass of a compound. Mass of benzene 75 g 100075. 9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. How to Calculate Number of Moles. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms.
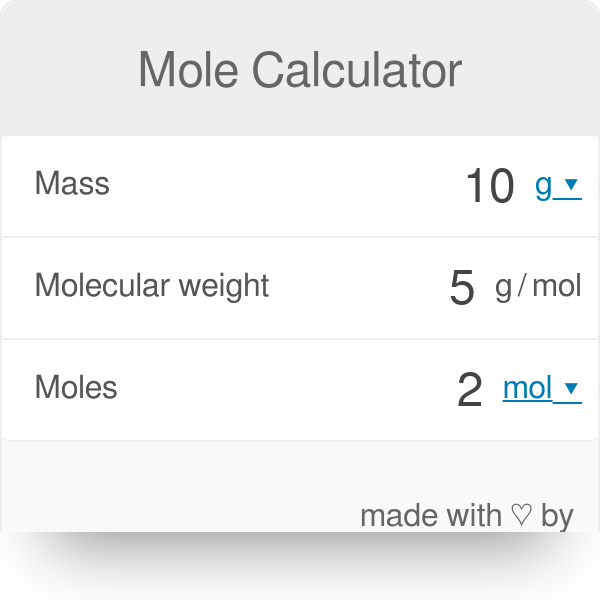 Source: omnicalculator.com
Source: omnicalculator.com
If you know a substance contains 3011 10 23 particles of the substance then the moles of substance will be 3011 10 23 6022 10 23 05 mol. From the equation 2 mol of. How to Find Moles. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. 25000 g of Cl2 are used and there are 70506 gmol of Cl2.
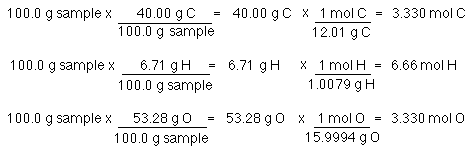 Source: westfield.ma.edu
Source: westfield.ma.edu
Glucose molecular formula is c 6 h 12 o 6. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. The characteristic molar mass of an element is simply the atomic mass in gmol. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. 9521 g has moles 1 mole.
 Source: youtube.com
Source: youtube.com
If you had 350 L of CO at STP then you would have 350 L 1 mol224 L 156 mol of CO Lastly it has been calculated that one mole of any substance contains 602 1023 atoms if you are talking about pure elements molecules if you are talking about covalent compounds or the general term particles used primarily for inorganic compounds. From the equation 2 mol of. 500 g of Na are used in this reaction and there are 22990 gmol. Discovering molar mass begins with models of grams per mole gmol. Determining Number of Moles of a Compound With Known Mass Once youve found the molecular weight you know the weight of one mole of a compound.
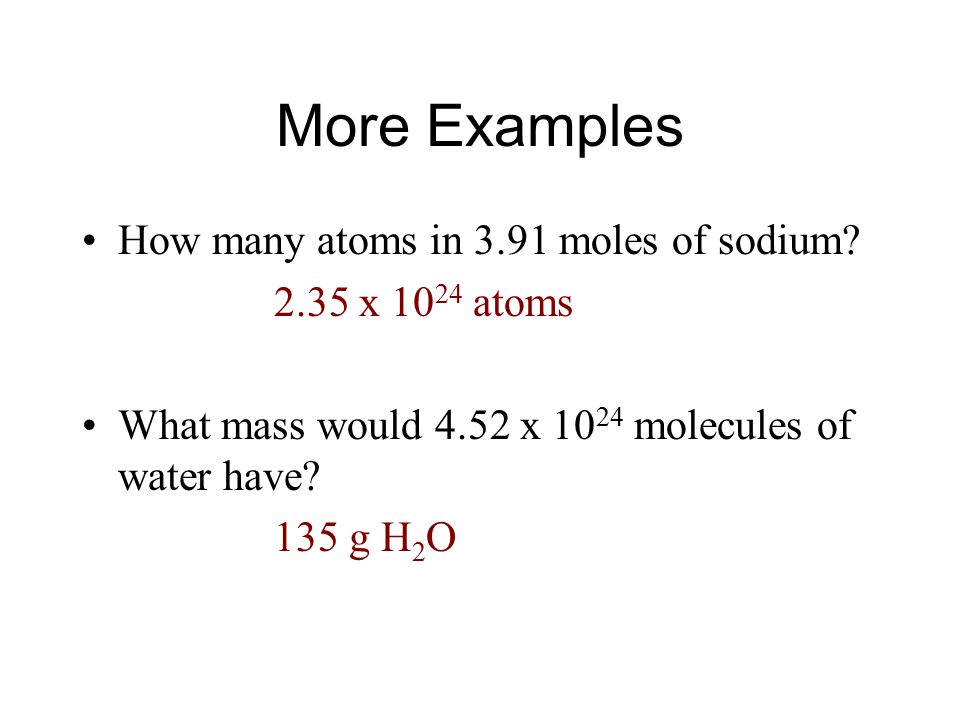 Source: slideplayer.com
Source: slideplayer.com
The answer is the number of. This chemistry video tutorial explains how to calculate the molar mass of a compound. Molecular mass 40078 x 3 3097361 x 2 159994 x 8 molecular mass 120234 6194722 1279952 molecular mass 31017642 from. 500 g of Na are used in this reaction and there are 22990 gmol. 9521 g has moles 1 mole.
 Source: youtube.com
Source: youtube.com
To do this you must need to know its chemical formula which is C X 9 H X 13 N O X 3. Jeremiah Daniels on How To Find Moles Of An Element In A Compound Given Mass. Divide the mass of the compound in grams by the molar mass you just calculated. NaCl Na Cl. Multiply the elements atomic mass by the number of atoms of that element in the compound.
 Source: youtube.com
Source: youtube.com
The characteristic molar mass of an element is simply the atomic mass in gmol. In other words it tells you the number of. So 7 g has moles 7 g X 1 mol 9521 gmol 00735 moles. It has units of grams per mole. We assume you are.
 Source: wikihow.com
Source: wikihow.com
The quotient is equal to the number of moles. What number of moles Iron in 1 grams. The molar mass is the combined atomic masses of the elements of the compound. This is the molar mass of the compound. To find the number of moles in a sample simply weigh it and divide the weight by the molecular weight.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate moles of compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






