Your How to calculate moles of atoms in a compound images are available. How to calculate moles of atoms in a compound are a topic that is being searched for and liked by netizens now. You can Find and Download the How to calculate moles of atoms in a compound files here. Find and Download all royalty-free images.
If you’re searching for how to calculate moles of atoms in a compound pictures information linked to the how to calculate moles of atoms in a compound keyword, you have pay a visit to the right blog. Our site always gives you hints for downloading the highest quality video and image content, please kindly search and find more enlightening video articles and images that fit your interests.
How To Calculate Moles Of Atoms In A Compound. For example the molar mass of NaCl can be calculated for finding the atomic mass of sodium 2299 gmol and the atomic mass. How do you find the number of atoms in a mole of a compound. For a compound with the molecular formula X a Y b. Converting Moles to Atoms.
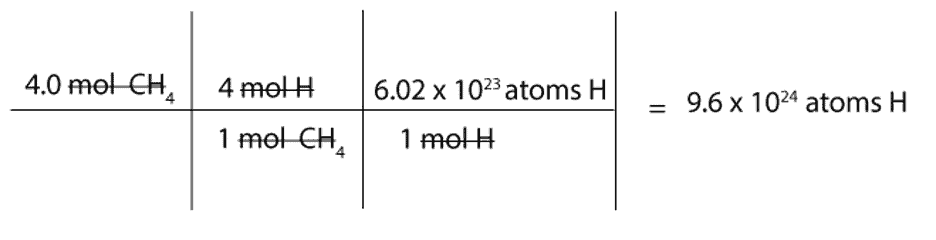 How To Interpret And Use Chemical Formula To Go From Moles Of One Substance To Moles Atoms Or Grams Of Another From masterconceptsinchemistry.com
How To Interpret And Use Chemical Formula To Go From Moles Of One Substance To Moles Atoms Or Grams Of Another From masterconceptsinchemistry.com
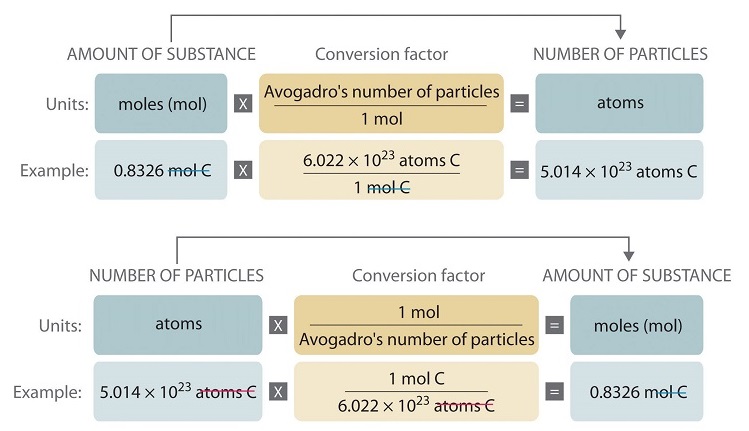
How many H atoms are there in 0046 g of C2H6OINTERVIEW1 Revell K. Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382. 1 mole of nitrogen atoms 14 g. For example lets take H X 2 element. So nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles. For example to convert between the number of atoms or other particles such as molecules in a sample and moles use the following conversion factor.
If you have 1000 grams.
Multiply your mole value by avogadros number 6021023. 1 molecule of compound X a Y b contains. For example 1 mol of sodium Na has a mass of 229898 g the mass on the periodic table. Calculating molar mass and number of moles. The mathematical equation N n N A can also be used to find the number of atoms of each element in a known amount in moles of a compound. What is a tefl qualification equivalent to.
 Source: youtube.com
Source: youtube.com
This chemistry video tutorial explains the conversion process of moles to atoms and how to convert the number of atoms to moles. Next multiply the atomic mass of each atom by the number of atoms in the compound. Given a known number of moles x one can find the number of atoms y in this molar quantity by multiplying it by Avogadros number. 1 mole 60221023 6022 10 23 atoms molecules protons etc. Skill 3-1 Calculate the molecular mass of a compound as the sum of the atomic masses of its elements.
 Source: youtube.com
Source: youtube.com
This tutorial contains plen. 5 moles H X 2 S O X 4. For a compound with the molecular formula X a Y b. As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms. It can be expressed as grams liters atoms molecules or particles.
 Source: youtube.com
Source: youtube.com
This tutorial contains plen. It can be expressed as grams liters atoms molecules or particles. How do you find the number of atoms in a mole of a compound. Molecular mass 1 x 140067 3 x 100794 molecular mass 140067 302382. A mole can be defined as the amount of substance.
 Source: slideplayer.com
Source: slideplayer.com
The same as the mass of one mole of the compound in grams. Then 1000 g 151001 gmol X g moles. We can then use the calculated molar mass to convert between mass and number of moles of the. In addition a mole of hydrogen is equal to a mole of glucose or a mole of uranium. The same as the mass of one mole of the compound in grams.
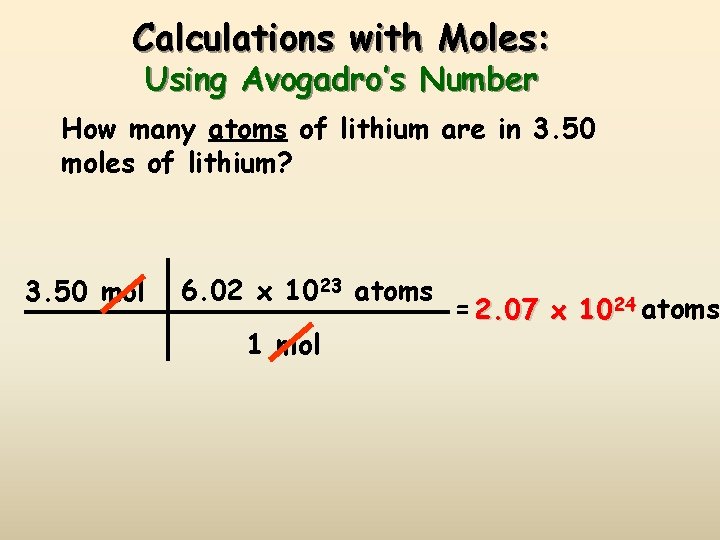 Source: slidetodoc.com
Source: slidetodoc.com
Multiply your mole value by avogadros number 6021023. X moles x 602 x 1023 SO3 molecules 1mol SO3 Y SO3 molecules. This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles. This tutorial contains plen. And 1 mol of chlorine Cl has a mass of 35453 g the mass on the periodic table.
 Source: youtube.com
Source: youtube.com
This is thoroughly answered here. Then 1000 g 151001 gmol X g moles. What is a tefl qualification equivalent to. Multiply your mole value by avogadros number 6021023. 10 moles H X 2 602 10 23 602 10 23 atoms.
 Source: slideshare.net
Source: slideshare.net
For example 1 mol of sodium Na has a mass of 229898 g the mass on the periodic table. This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles. 1 mole of nitrogen atoms 14 g. This is thoroughly answered here. How many moles of hydrogen atoms are there in 0046 g of C2H6O.
 Source: khanacademy.org
Source: khanacademy.org
In addition a mole of hydrogen is equal to a mole of glucose or a mole of uranium. How many atoms in 55 moles. In grams a mole is one formula mass. How many atoms of each element are found in. This chemistry video tutorial explains the conversion process of moles to atoms and how to convert the number of atoms to moles.
 Source: slideplayer.com
Source: slideplayer.com
How many moles of hydrogen atoms are there in 0046 g of C2H6O. This tutorial contains plen. Peach smoothie bowl without yogurt. As shown in this video we can obtain a substances molar mass by summing the molar masses of its component atoms. A moles of.
 Source: youtube.com
Source: youtube.com
How many atoms in 55 moles. A mole can be defined as the amount of substance. Also it is easier to calculate atoms in a mole than in lakhs and crores. There are three hydrogen atoms as indicated by the subscript. Home connect home assistant.
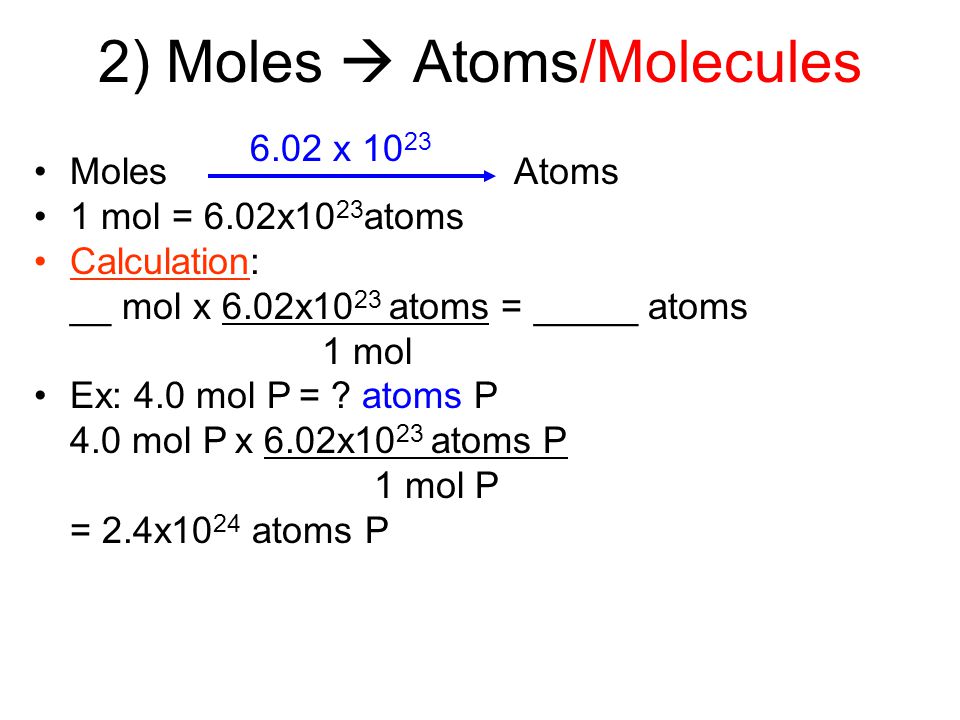 Source: slideplayer.com
Source: slideplayer.com
1 molecule of compound X a Y b contains. It can be expressed as grams liters atoms molecules or particles. Next multiply the atomic mass of each atom by the number of atoms in the compound. A atoms of element X. The mathematical equation N n N A can also be used to find the number of atoms of each element in a known amount in moles of a compound.
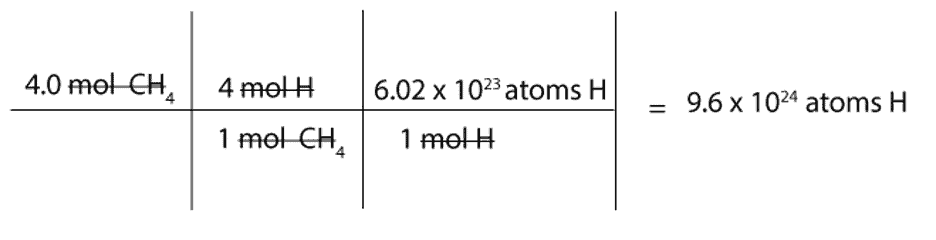 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
To calculate the molar mass of a compound with multiple atoms sum all the atomic mass of the constituent atoms. Avogadros number is a very important relationship to remember. Converting Moles to Atoms. You calculate the number of moles by dividing the mass of substance by the substances atomic or molecular weight. 1 mole of compound X a Y b contains.
 Source: chem.libretexts.org
Source: chem.libretexts.org
How many atoms of each element are found in. Best hikes near crystal mountain. We can then use the calculated molar mass to convert between mass and number of moles of the. Next multiply the atomic mass of each atom by the number of atoms in the compound. 1 molecule of compound X a Y b contains.
 Source: youtube.com
Source: youtube.com
This is thoroughly answered here. Moles are units used to measure substance amount. Now the only confusing thing for me is the 5 moles. Then 1000 g 151001 gmol X g moles. We can then use the calculated molar mass to convert between mass and number of moles of the.
 Source: thefactfactor.com
Source: thefactfactor.com
For example lets take H X 2 element. In addition a mole of hydrogen is equal to a mole of glucose or a mole of uranium. So nNNa – n Na N number of atoms 2 1grmol H 2 moles H x 5 moles in the compound 10 moles. The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. How many atoms in 55 moles.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate moles of atoms in a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






