Your How to calculate moles of anhydrous compound images are available in this site. How to calculate moles of anhydrous compound are a topic that is being searched for and liked by netizens today. You can Get the How to calculate moles of anhydrous compound files here. Get all free photos.
If you’re searching for how to calculate moles of anhydrous compound images information connected with to the how to calculate moles of anhydrous compound interest, you have pay a visit to the ideal site. Our website always gives you suggestions for refferencing the highest quality video and image content, please kindly hunt and locate more enlightening video articles and images that fit your interests.
How To Calculate Moles Of Anhydrous Compound. Magnesium chloride anhydrous weighs 232 gram per cubic centimeter or 2 320 kilogram per cubic meter ie. The mass of water lost during heating can be determined and the coefficient. Hydrate —— anhydrous salt water. 431 322 109 g of water.
 What Is An Anhydrous Compound Use The Following Expe Techwhiff From techwhiff.com
What Is An Anhydrous Compound Use The Following Expe Techwhiff From techwhiff.com
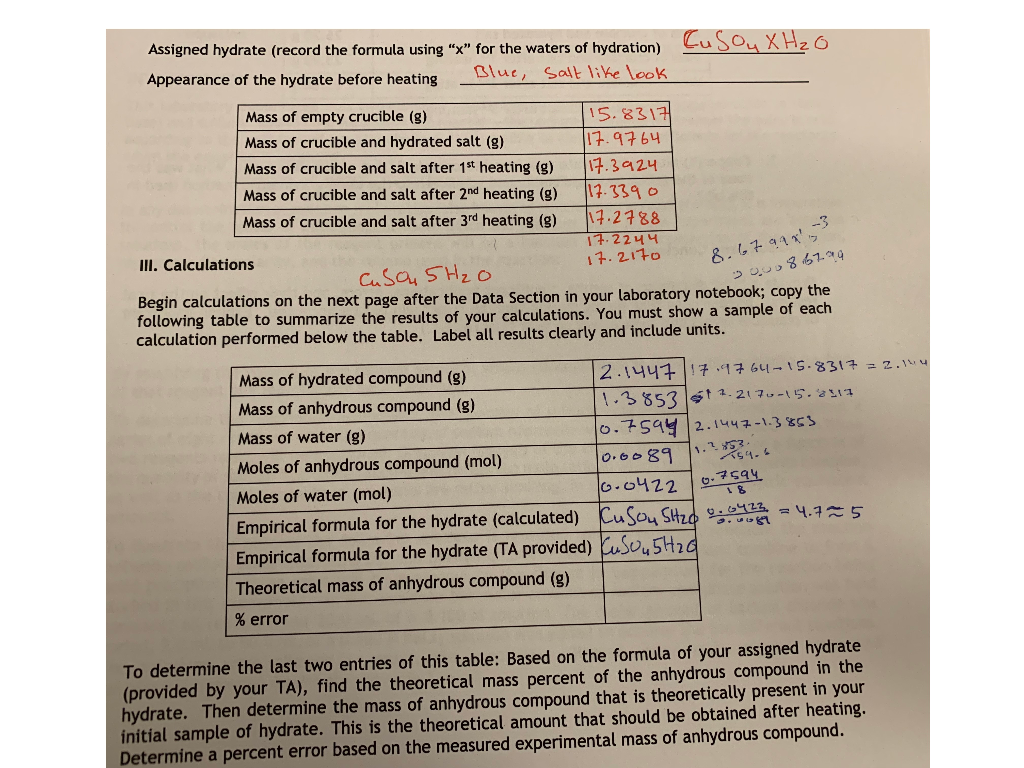
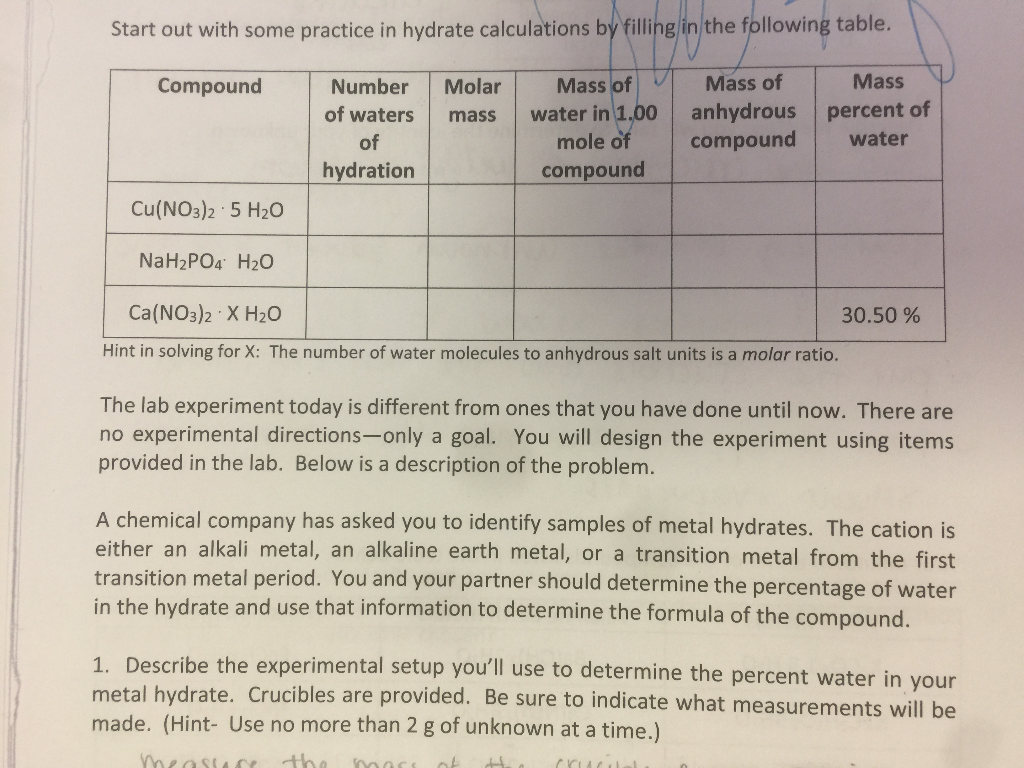
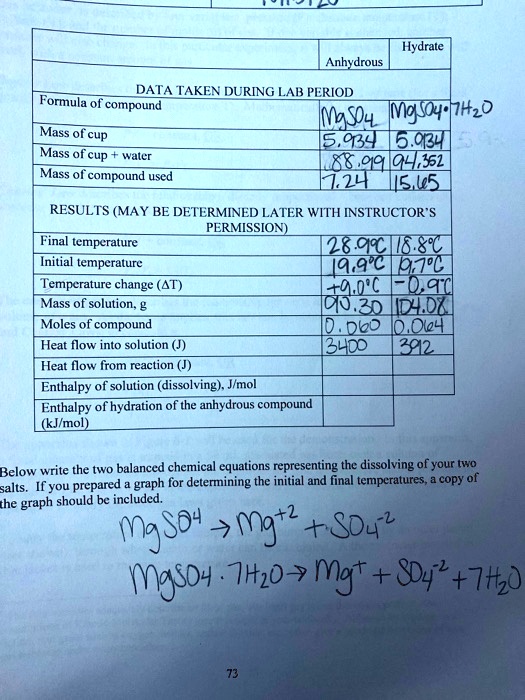
Based on the correct answer to question 7 calculate the ratio of moles of H2O to moles of anhydrous CuSO4 if there were 0085 mols of released H2O. Convert the mass of the anhydrous salt m2 by dividing it by the molar mass of the anhydrous salt. Substract the Constant Weight of the Crucible and Anhydrate from the Mass of the Crucible and contents before heating 21. The mass of water lost during heating can be determined and the coefficient. Determine the formula of the hydrate and then write out the name of the hydrate. In other words the if 4875g of the hydrated compound is used.
Calculate the number of moles of anhydrate.
011 moles H 2 O 0018 moles FeCl 3 611. Mole ratio moles of water moles of anhydrate. In other words the if 4875g of the hydrated compound is used. The same notation applies for water. N A - number of moles of anhydrous compound. About Magnesium chloride anhydrous.
 Source: chegg.com
Source: chegg.com
In other words the if 4875g of the hydrated compound is used. Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. The same notation applies for water. Density of magnesium chloride anhydrous is equal to 2 320 kgm³. In our example 16 grams 160 grams per mole 01 moles.
 Source: chegg.com
Source: chegg.com
In other words the if 4875g of the hydrated compound is used. 260 g H2 O 1800 gmol H2 O 0144 moles H2 O. Well call this 6. Calculate the moles of anhydrous CuSO4 present in a sample with 05 g CuSO4 at a molar mass of 15961 gmol. Na 2 CO 3— 322 g 105988 gmol 00304 mol.
 Source: chegg.com
Source: chegg.com
M A - mass of anhydrous compound. Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. 2 Determine moles of Na 2 CO 3 and water. The number of moles of anhydrous compound CuSO4 is. 133 g 001055 mol 126066 gmol — the molar mass of H2C2O4nH2O.
 Source: techwhiff.com
Source: techwhiff.com
Based on the correct answer to question 7 calculate the ratio of moles of H2O to moles of anhydrous CuSO4 if there were 0085 mols of released H2O. Divide the mass of water lost when you heated the salt by the molar mass of water roughly 18 grams per mole. It contains plenty of examples and practice problemsMy E-Book. The formula mass of the water is 4505g. 1 Determine mass of water driven off.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Molar mass of COCl2anhydrous is 989161 gmol Convert between COCl2anhydrous weight and moles. 011 moles H 2 O 0018 moles FeCl 3 611. Convert this to moles of water by dividing by the molar mass of water which is 1802 gmol. About Magnesium chloride anhydrous. Salt and can be removed upon heating yielding the anhydrous salt salt without water.

The attempt at a solution. The attempt at a solution. 1 Determine mass of water driven off. Click to see full answer. In chemical formula you may use.
 Source: chegg.com
Source: chegg.com
M A - molar mass of anhydrous compound. It contains plenty of examples and practice problemsMy E-Book. Convert this to moles of water by dividing by the molar mass of water which is 1802 gmol. Density of magnesium chloride anhydrous is equal to 2 320 kgm³. The same notation applies for water.
 Source: chegg.com
Source: chegg.com
In our example 16 grams 160 grams per mole 01 moles. The first thing to do is to determine the moles of water that were in the sample. 133 g 001055 mol 126066 gmol — the molar mass of H2C2O4nH2O. Divide the mass of water by the molar mass of water to get moles of water. Lastly to find the number of moles of water per mole of hydrate I divided the number of moles of anhydrous salt remaining by itself and by the number of moles of water lost.
 Source: chegg.com
Source: chegg.com
Divided the grams of anhydrate by its molar mass to find the number of moles 20. Lastly to find the number of moles of water per mole of hydrate I divided the number of moles of anhydrous salt remaining by itself and by the number of moles of water lost. In our example 16 grams 160 grams per mole 01 moles. At 20C 68F or 29315K at standard atmospheric pressureIn Imperial or US customary measurement system the density is equal to 144833 pound per cubic foot. 1 Determine mass of water driven off.
 Source: chegg.com
Source: chegg.com
Determine the number of grams of water which escaped from the crucible. Cu 1 6355 6355. M A - molar mass of anhydrous compound. Mw m1 - m2. 126066 900338 360322 g —.
 Source: studylib.net
Source: studylib.net
The number of moles of anhydrous compound CuSO4 is. Enter a chemical formula to calculate its molar mass and elemental composition. O 6 1600 9600. Based on the correct answer to question 7 calculate the ratio of moles of H2O to moles of anhydrous CuSO4 if there were 0085 mols of released H2O. At 20C 68F or 29315K at standard atmospheric pressureIn Imperial or US customary measurement system the density is equal to 144833 pound per cubic foot.
 Source: chegg.com
Source: chegg.com
In our example 16 grams 160 grams per mole 01 moles. Ca Fe Mg Mn S O H C N Na K Cl Al. X 001055 mol — this is how many moles of H2C2O4nH2O dissolved in the 2500 mL. About Magnesium chloride anhydrous. N 2 1401 2802.
 Source: youtube.com
Source: youtube.com
61 is very close to 6 so it is safe to round down. Well call this 6. The mass of water lost during heating can be determined and the coefficient. 2 Determine moles of Na 2 CO 3 and water. Divided the grams of anhydrate by its molar mass to find the number of moles 20.
 Source: numerade.com
Source: numerade.com
The formula mass of the water is 4505g. The first thing to do is to determine the moles of water that were in the sample. The formula mass of the water is 4505g. Molar mass of COCl2anhydrous is 989161 gmol Convert between COCl2anhydrous weight and moles. O 6 1600 9600.
 Source: numerade.com
Source: numerade.com
Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. 133 g 001055 mol 126066 gmol — the molar mass of H2C2O4nH2O. N A - number of moles of anhydrous compound. The attempt at a solution. X 001055 mol — this is how many moles of H2C2O4nH2O dissolved in the 2500 mL.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate moles of anhydrous compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






