Your How to calculate moles in compound images are available. How to calculate moles in compound are a topic that is being searched for and liked by netizens today. You can Download the How to calculate moles in compound files here. Find and Download all royalty-free images.
If you’re searching for how to calculate moles in compound images information related to the how to calculate moles in compound topic, you have pay a visit to the ideal site. Our website always gives you suggestions for downloading the maximum quality video and picture content, please kindly search and locate more enlightening video content and graphics that match your interests.
How To Calculate Moles In Compound. Ions have oxidation numbers equal to their charge. Molar mass Mg 243 gmol You have determined that the. Calculate he variety of moles you could have by taking the Mass molar mass. Then 1000 g 151001 gmol X g.
 Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy From khanacademy.org
Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy From khanacademy.org
Number of moles Mass of substance Mass of one mole. O - 300495 mol 0148 mol. Use the molar mass calculator to easily get an accurate set of data describing any compound. Molecular mass 40078 x 3 3097361 x 2 159994 x 8 molecular mass 120234 6194722 1279952. If your compound were potassium sulfide K₂S you would add the mass of 2 mol of potassium 2 39097 g and the mass of 1 mol of sulfur 32064 g. For example one molecule of H2O has two atoms of Hydrogen H.
Numerically this would be 2 1008 1 1600 18016.
How to Calculate Number of Moles. Molar mass Mg 243 gmol You have determined that the. This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles. Moles of element n moles element one mole of compound K - 1 mole of K for 1 mole of KNO3. How to Find Moles. For example one molecule of H2O has two atoms of Hydrogen H.
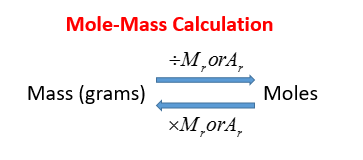 Source: onlinemathlearning.com
Source: onlinemathlearning.com
9061022 x 1 mol NH4 60221023 0150 mol NH4 -this is moles of atoms in the positive NH4 ion. Apr 30 2018 Chemists use moles. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. One mole of a compound contains Avogadros number 6022 x 10 23 of molecules molecular compound or formula units ionic compoundThe molar mass of a compound tells you the mass of 1 mole of that substance. M r of Na 2 SO 4 23 23 32 16 16 16 16 142.
 Source: wikihow.com
Source: wikihow.com
The quotient is equal to the number of moles. M r of NaOH 23 16 1 40. If you have 1000 grams. Use the molar mass calculator to easily get an accurate set of data describing any compound. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom.
 Source: chem.fsu.edu
Source: chem.fsu.edu
NaCl Na Cl. Numerically this would be 2 1008 1 1600 18016. To go from grams to moles divide the grams by the molar mass. Lets start with these values. Calculate Mole In Compoundto scientific research in any way.
 Source: surfguppy.com
Source: surfguppy.com
This would be the mass of 1 mol of K₂S. To do that you need to must know its chemical system which is C X 9 H X 13. K - 00495 mol. Mass of MnO2 95g. Apr 30 2018 Chemists use moles.
 Source: slideplayer.com
Source: slideplayer.com
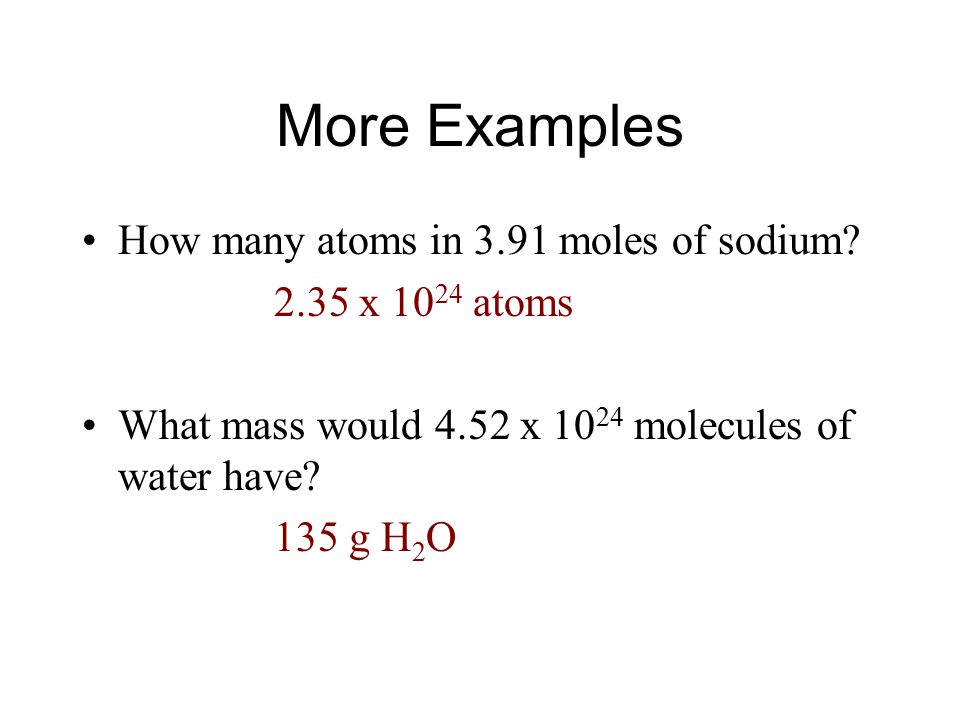
N 1 mole of N for 1 mole of KNO3. To do that you need to must know its chemical system which is C X 9 H X 13. N the number of moles of a compound. Molar mass Mg 243 gmol You have determined that the. 200 mL H 2 O.
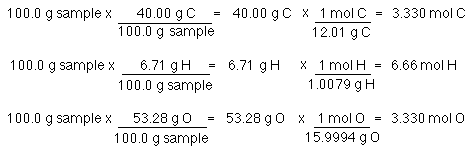 Source: westfield.ma.edu
Source: westfield.ma.edu
Molar mass Mg 243 gmol You have determined that the. Molecular mass 40078 x 3 3097361 x 2 159994 x 8 molecular mass 120234 6194722 1279952. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. To convert between grams and moles you would use the substances molar mass. Also how many moles are in a gram.
 Source: youtube.com
Source: youtube.com
NaCl Na Cl. From the equation 2 mol of. 200 mL H 2 O. How do you calculate molarity. Answer 1 of 2.
 Source: youtube.com
Source: youtube.com
From compound to compound to a number of atoms in a molecule vary. Fifth convert to grams. Use the molar mass calculator to easily get an accurate set of data describing any compound. Following the same process outlined above we can determine the molarity of this calcium chloride solution in a few simple steps. N - 00495 mol.
 Source: youtube.com
Source: youtube.com
Number of moles of NaOH mass relative formula mass 20 40 05 mol. The final answer uses the correct number of significant figures. Number of moles formula is. How to Calculate Number of Moles. To go from grams to moles divide the grams by the molar mass.
 Source: slideplayer.com
Source: slideplayer.com
Number of moles 95 8694. If you have 1000 grams. How to Calculate Number of Moles. Number of moles 1092 mol. The characteristic molar mass of an element is simply the atomic mass in gmol.
 Source: khanacademy.org
Source: khanacademy.org
Using the formula molesmassmolar mass i found how to calculate molar mass using the formula molar massmolesmasstherefore i was under the impression that surely 602times10 23 divided by the mass of the compound would have resulted in the molar mass. O - 300495 mol 0148 mol. K - 00495 mol. So The number of moles of each element. NaCl 229898 gL 354530 gL.
 Source: slideshare.net
Source: slideshare.net
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. The characteristic molar mass of an element is simply the atomic mass in gmol. Mass of one mole MnO2 8694g. Divide the mass of the compound in grams by the molar mass you just calculated. M r of NaOH 23 16 1 40.
 Source: dummies.com
Source: dummies.com
The periodic table provides the atomic masses that are used to calculate molar mass. M r of Na 2 SO 4 23 23 32 16 16 16 16 142. 1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 10 22 ions and the number of moles of NH4 ions 00301 moles. M r of NaOH 23 16 1 40. Also how many moles are in a gram.
 Source: youtube.com
Source: youtube.com
Divide the mass of the compound in grams by the molar mass you just calculated. 100 g CaCl 2. 1811022 x 5 there are 5 atoms in NH4 9061022 Nmber of NH4 ions 181 x 10 22 ions and the number of moles of NH4 ions 00301 moles. Number of moles 1092 mol. So The number of moles of each element.
 Source: youtube.com
Source: youtube.com
So The number of moles of each element. Divide the mass of the compound in grams by the molar mass you just calculated. Number of moles of compound n molar massmass. Number of moles formula is. Molecular mass 40078 x 3 3097361 x 2 159994 x 8 molecular mass 120234 6194722 1279952.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate moles in compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






