Your How to calculate moles from grams of a compound images are available in this site. How to calculate moles from grams of a compound are a topic that is being searched for and liked by netizens now. You can Find and Download the How to calculate moles from grams of a compound files here. Find and Download all free images.
If you’re searching for how to calculate moles from grams of a compound images information linked to the how to calculate moles from grams of a compound topic, you have visit the right site. Our site frequently gives you hints for seeing the highest quality video and picture content, please kindly surf and locate more enlightening video articles and images that match your interests.
How To Calculate Moles From Grams Of A Compound. We assume you are converting between moles NaOH and gram. The formula for moles to grams is given by. One mole consists of Avogadro number of atoms. This converts atomic units to grams per mole making the molar mass of hydrogen 1007 grams per mole of carbon 120107 grams per mole of oxygen 159994 grams per mole and of chlorine 35453 grams per mole.
 How To Convert Grams To Moles Video Lesson Transcript Study Com From study.com
How To Convert Grams To Moles Video Lesson Transcript Study Com From study.com
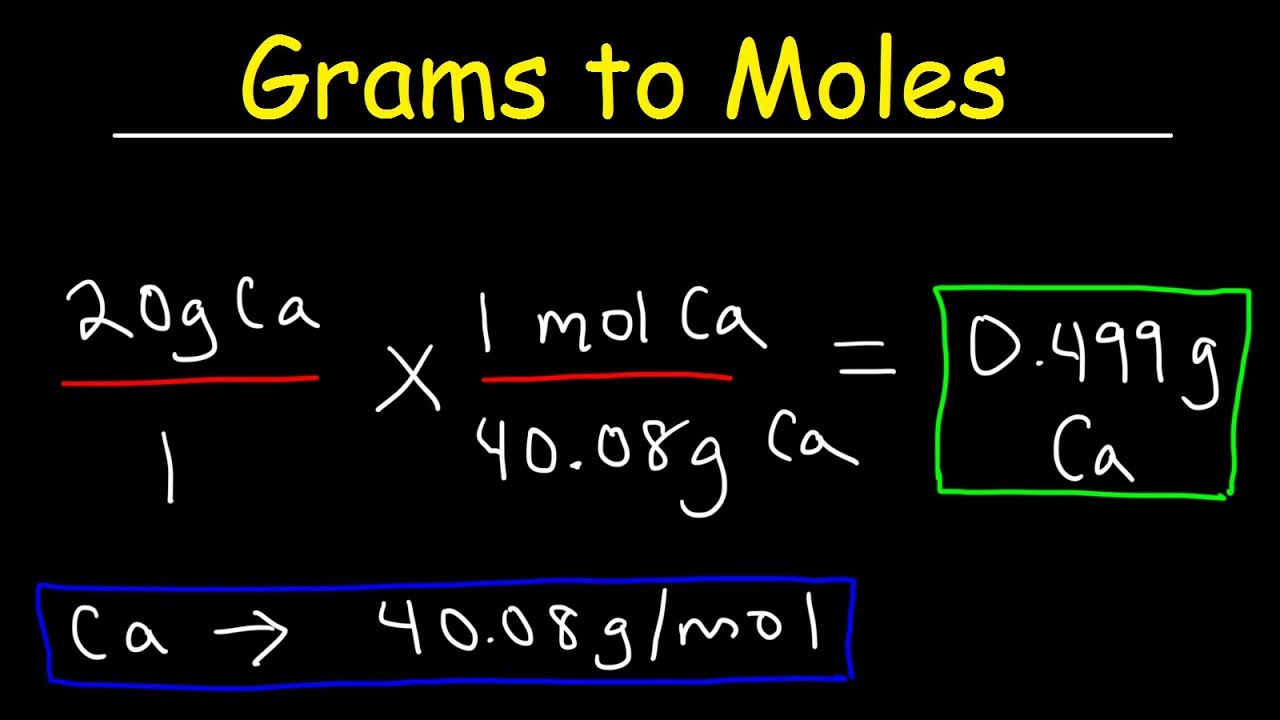
We assume you are converting between moles Sulfur and gram. How many moles Sulfur in 1 grams. The answer is 0025001806380511. Look for the atomic masses of hydrogen sulfur and oxygen. This is defined as 0001 kilogram per mole or 1 gram per mole. One mole consists of Avogadro number of atoms.
The answer is 0031186652112896.
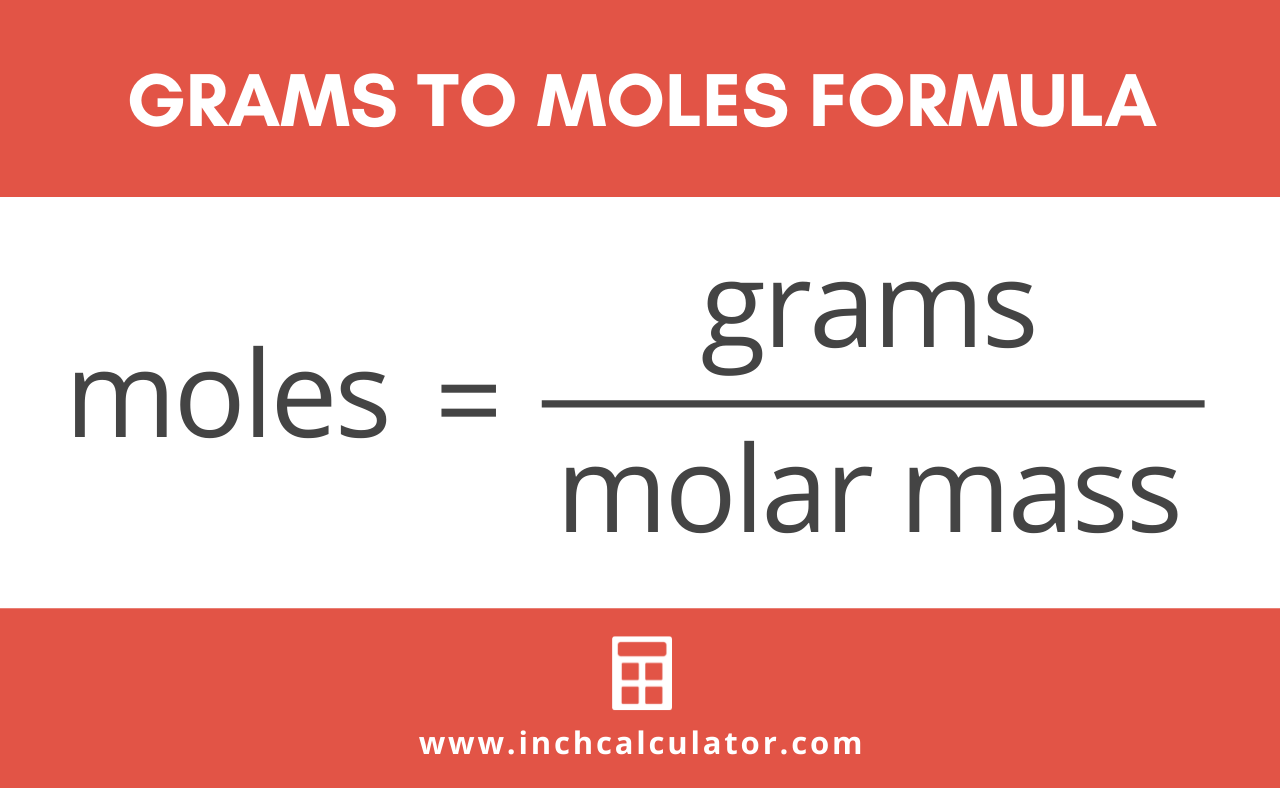
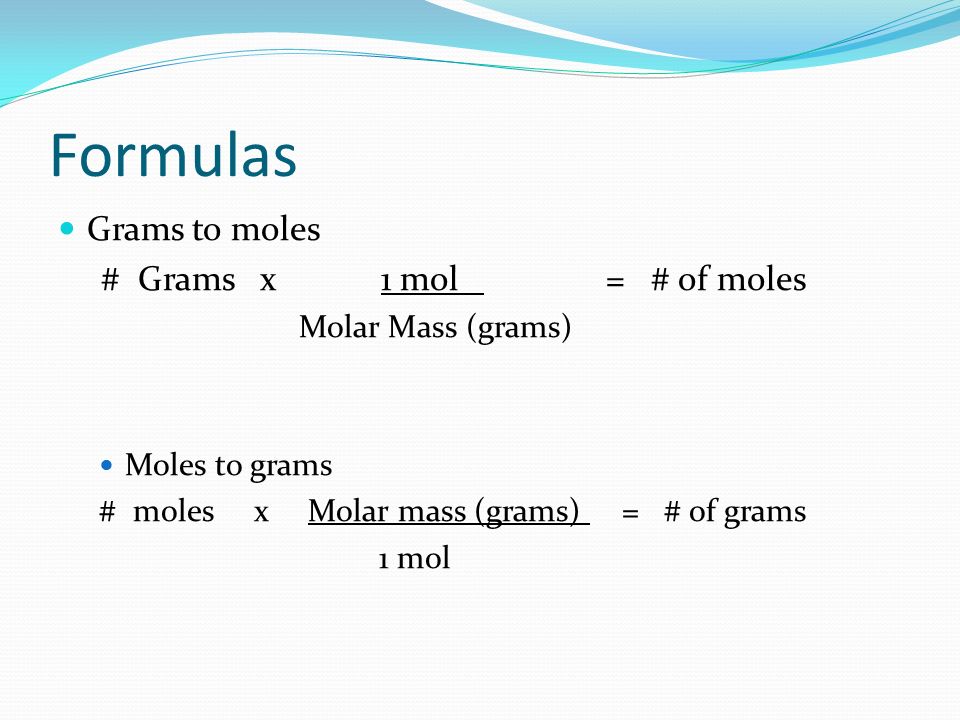
If you know the quantity of mole it can be converted into grams and vice versa. The formula for moles to grams is given by. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. The SI base unit for amount of substance is the mole. Molecular weight of Sulfur or grams The molecular formula for Sulfur is S. You can view more details on each measurement unit.
 Source: inchcalculator.com
Source: inchcalculator.com
The formula for moles to grams is given by. The SI base unit for amount of substance is the mole. How many moles NaOH in 1 grams. This is defined as 0001 kilogram per mole or 1 gram per mole. You can view more details on each measurement unit.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. Look for the atomic masses of hydrogen sulfur and oxygen. You can view more details on each measurement unit. One mole consists of Avogadro number of atoms.
 Source: study.com
Source: study.com
Molecular weight of NaOH or grams This compound is also known as Sodium Hydroxide. Some elements are only found in molecules of 2 atoms or more. This is defined as 0001 kilogram per mole or 1 gram per mole. Molecular weight of Sulfur or grams The molecular formula for Sulfur is S. We assume you are converting between moles Sulfur and gram.
 Source: youtube.com
Source: youtube.com
Some elements are only found in molecules of 2 atoms or more. Molecular weight of Sulfur or grams The molecular formula for Sulfur is S. The SI base unit for amount of substance is the mole. If you know the quantity of mole it can be converted into grams and vice versa. The answer is 0031186652112896.
 Source: slideplayer.com
Source: slideplayer.com
You can view more details on each measurement unit. One mole consists of Avogadro number of atoms. How many moles NaOH in 1 grams. Some elements are only found in molecules of 2 atoms or more. If you know the quantity of mole it can be converted into grams and vice versa.
 Source: youtube.com
Source: youtube.com
How many moles Sulfur in 1 grams. If you know the quantity of mole it can be converted into grams and vice versa. We assume you are converting between moles NaOH and gram. The answer is 0025001806380511. One mole consists of Avogadro number of atoms.
 Source: wikihow.com
Source: wikihow.com
You can view more details on each measurement unit. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. If you know the quantity of mole it can be converted into grams and vice versa. You can view more details on each measurement unit. Molecular weight of Sulfur or grams The molecular formula for Sulfur is S.
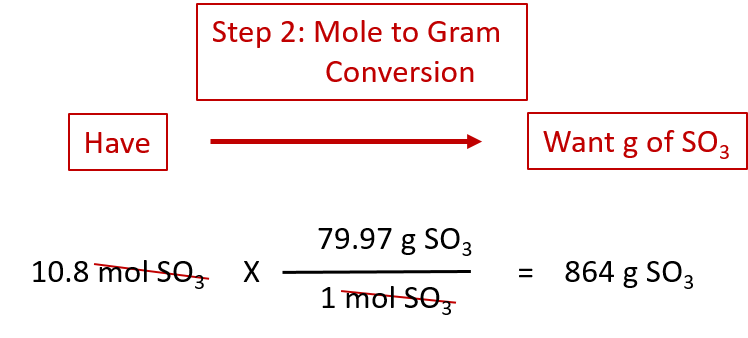 Source: wou.edu
Source: wou.edu
The SI base unit for amount of substance is the mole. This is defined as 0001 kilogram per mole or 1 gram per mole. How many moles NaOH in 1 grams. Molecular weight of NaOH or grams This compound is also known as Sodium Hydroxide. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4.
 Source: youtube.com
Source: youtube.com
One mole consists of Avogadro number of atoms. The SI base unit for amount of substance is the mole. This is defined as 0001 kilogram per mole or 1 gram per mole. 1 mole is equal to 1 moles Sulfur or. Look for the atomic masses of hydrogen sulfur and oxygen.
 Source: pinterest.com
Source: pinterest.com
The answer is 0031186652112896. The answer is 0025001806380511. This is defined as 0001 kilogram per mole or 1 gram per mole. How many moles Sulfur in 1 grams. Molecular weight of Sulfur or grams The molecular formula for Sulfur is S.
 Source: khanacademy.org
Source: khanacademy.org
The answer is 0031186652112896. Look for the atomic masses of hydrogen sulfur and oxygen. We assume you are converting between moles Sulfur and gram. Molecular weight of NaOH or grams This compound is also known as Sodium Hydroxide. One mole consists of Avogadro number of atoms.
 Source: study.com
Source: study.com
The answer is 0031186652112896. Molecular weight of NaOH or grams This compound is also known as Sodium Hydroxide. Example 1 Calculate the mass in grams of 36 mol of H 2 SO 4. The answer is 0025001806380511. This is defined as 0001 kilogram per mole or 1 gram per mole.
 Source: study.com
Source: study.com
Some elements are only found in molecules of 2 atoms or more. This is defined as 0001 kilogram per mole or 1 gram per mole. One mole consists of Avogadro number of atoms. If you know the quantity of mole it can be converted into grams and vice versa. Some elements are only found in molecules of 2 atoms or more.
 Source: wikihow.com
Source: wikihow.com
The answer is 0025001806380511. The formula for moles to grams is given by. How many moles Sulfur in 1 grams. The answer is 0025001806380511. The answer is 0031186652112896.
 Source: youtube.com
Source: youtube.com
The answer is 0025001806380511. Molecular weight of NaOH or grams This compound is also known as Sodium Hydroxide. The SI base unit for amount of substance is the mole. Some elements are only found in molecules of 2 atoms or more. The SI base unit for amount of substance is the mole.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate moles from grams of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.