Your How to calculate mole ratio of a compound images are available in this site. How to calculate mole ratio of a compound are a topic that is being searched for and liked by netizens today. You can Download the How to calculate mole ratio of a compound files here. Get all royalty-free images.
If you’re looking for how to calculate mole ratio of a compound images information connected with to the how to calculate mole ratio of a compound topic, you have visit the right site. Our website frequently gives you suggestions for downloading the highest quality video and picture content, please kindly hunt and find more enlightening video articles and images that fit your interests.
How To Calculate Mole Ratio Of A Compound. Moles H 458 g H x 1 mol H 101 g H 453 mol H. Therefore if we know the mass of an element we can calculate how many atoms of that element are present. For example the ratio for the amount of nitrogen in two moles of nitrogen dioxide 2NO2 is 1-to-2. 2 mol H 2 O.
 Mole Review 1 Calculate The Number Of Moles From slidetodoc.com
Mole Review 1 Calculate The Number Of Moles From slidetodoc.com
We identified it from well-behaved source. Mass percent grams of solute grams of solute plus solvent x 100. To find the simplest whole number ratio divide each number by the smallest number of moles. Mass percent mass of element in 1 mole of compound mass of 1 mole of compound x 100. The mole ratio can be known as the mole-to. 120g Al 1 mol Al 2698g Al 0044 48 mol Al.
For each compound find the grams of copper that combine with 100 g of chlorine by dividing the mass of copper by the mass of chlorine.
Here are a number of highest rated How To Calculate Mole Ratio pictures upon internet. We identified it from well-behaved source. For each one mole of CuSO 4 there are 5 moles of water. Therefore if we know the mass of an element we can calculate how many atoms of that element are present. Calculate the molar ratios. In other words 1 mol of methane will produced 1 mole of carbon dioxide as long as the reaction goes to completion and there is plenty of oxygen present.
 Source: youtube.com
Source: youtube.com
To find the simplest whole number ratio divide each number by the smallest number of moles. Its submitted by admin in the best field. This quantity is called Avogadros number. That means that answer choice a would be considered by most teachers to be the correct answer. However this reduces to a 11 ratio.
 Source: quizlet.com
Source: quizlet.com
Moles H 458 g H x 1 mol H 101 g H 453 mol H. For example A compound containing only Mn and Cl contains 19228 g Mn and 24817 g Cl. Mass percent grams of solute grams of solute plus solvent x 100. Draw a line vertically from that composition to the bubble point or liquid line. Lets say you have a mixture of components 1 and 2 that is 40 mole percent mole fraction 04 component one approximately the blue line labeled liquid on the diagram.
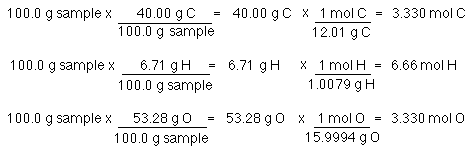 Source: westfield.ma.edu
Source: westfield.ma.edu
In chemistry the molar mass of a chemical compound is defined as the mass of 1 mole or 60221410 23 particles of the substance expressed in grams. For example - analysis of a sample of water would reveal that the number of moles of hydrogen atoms present in the sample is twice the number of moles of oxygen atoms in the sample. Feb 09 2021 Steps to find out empirical system. Mass to Mole Calculator - ezcalcme tip ezcalcme. Mass percent mass of element in 1 mole of compound mass of 1 mole of compound x 100.
 Source: slideplayer.com
Source: slideplayer.com
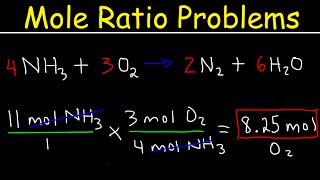
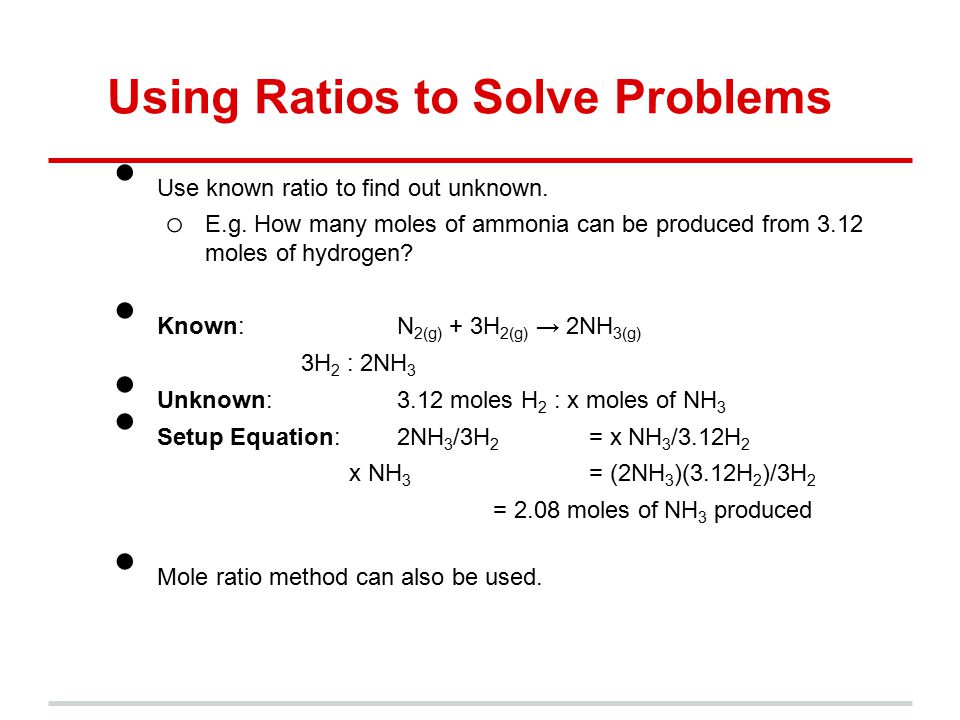
One mole is defined as eq6times 10 23 eq particles. 2 mol O 2. From the coefficients of the equation the mole ratio is 33. Convert all masses into moles. If there are methyl groups in both raw materials and products the CH3 signal integral ratio of the two is the molar ratio of the two.
 Source: dummies.com
Source: dummies.com
However this reduces to a 11 ratio. Mass percentage is calculated as the mass of a component divided by the total mass of the mixture multiplied by 100. Well if C12 and O16 how many moles of Carbon atoms are in 25g of CO2 Molar mass 44g so 44g of CO2 contains 1 mole of atoms 12g of C and 2 moles of atoms 32 g of O So 25g of CO2 will contain 144 x 25 moles of C atoms and 244 x 25 moles of O atoms. Apply this to your problem. Draw a line vertically from that composition to the bubble point or liquid line.
 Source: slideplayer.com
Source: slideplayer.com
We allow this nice of How To Calculate Mole Ratio graphic could possibly be the most trending topic bearing in mind we share it in google gain or facebook. Well if C12 and O16 how many moles of Carbon atoms are in 25g of CO2 Molar mass 44g so 44g of CO2 contains 1 mole of atoms 12g of C and 2 moles of atoms 32 g of O So 25g of CO2 will contain 144 x 25 moles of C atoms and 244 x 25 moles of O atoms. The ratio between them is the molar ratio. The characteristic molar mass of an element is simply the atomic mass in gmol. Mass percentage is calculated as the mass of a component divided by the total mass of the mixture multiplied by 100.
 Source: nagwa.com
Source: nagwa.com
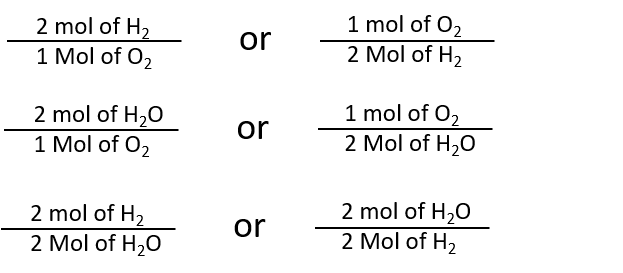
For each one mole of CuSO 4 there are 5 moles of water. 2 mol H 2 O. For each compound find the grams of copper that combine with 100 g of chlorine by dividing the mass of copper by the mass of chlorine. Apply this to your problem. For the reaction2 H2g O2g 2 H2Og The mole ratio between O2 and H2O is 12.
 Source: youtube.com
Source: youtube.com
Mass percent grams of solute grams of solute plus solvent x 100. Convert all masses into moles. However this reduces to a 11 ratio. Mass percent grams of solute grams of solute plus solvent x 100. The mole ratio can be known as the mole-to.
 Source: bulbapp.com
Source: bulbapp.com
In other words 1 mol of methane will produced 1 mole of carbon dioxide as long as the reaction goes to completion and there is plenty of oxygen present. 2 mol H 2 O. For the reaction2 H2g O2g 2 H2Og The mole ratio between O2 and H2O is 12. We identified it from well-behaved source. Use every parts molar mass to transform the grams of every ingredient to moles.
 Source: slideplayer.com
Source: slideplayer.com
That means that answer choice a would be considered by most teachers to be the correct answer. Please note that using a 33 ratio in a calculation is equivalent to using a 11 ratio. That means that answer choice a would be considered by most teachers to be the correct answer. These molar ratios can also be expressed as fractions. From the coefficients of the equation the mole ratio is 33.
 Source: slidetodoc.com
Source: slidetodoc.com
The characteristic molar mass of an element is simply the atomic mass in gmol. Please note that using a 33 ratio in a calculation is equivalent to using a 11 ratio. Mass percent grams of solute grams of solute plus solvent x 100. For example A compound containing only Mn and Cl contains 19228 g Mn and 24817 g Cl. To find the simplest whole number ratio divide each number by the smallest number of moles.
 Source: pinterest.com
Source: pinterest.com
Then draw a horizontal isotherm at that point red line on the diagram above. Please note that using a 33 ratio in a calculation is equivalent to using a 11 ratio. However this reduces to a 11 ratio. In chemistry the molar mass of a chemical compound is defined as the mass of 1 mole or 60221410 23 particles of the substance expressed in grams. Apply the law of multiple proportions to the two compounds.
 Source: slideplayer.com
Source: slideplayer.com
Use the molar mass calculator to easily get an accurate set of data describing any compound. Mass percentage is calculated as the mass of a component divided by the total mass of the mixture multiplied by 100. 2 mol H 2 O. Moles O 545 g O x 1 mol O 1600 g O 341 mol O. Lets say you have a mixture of components 1 and 2 that is 40 mole percent mole fraction 04 component one approximately the blue line labeled liquid on the diagram.
 Source: wou.edu
Source: wou.edu
How to convert mass to mole. Write the number of moles of each element over the number of moles of the compound. We identified it from well-behaved source. Here are a number of highest rated How To Calculate Mole Ratio pictures upon internet. If you want to calculate the mole ratio as a prove you can use the mass weight ratio or atomic ratio.
 Source: youtube.com
Source: youtube.com
Here are a number of highest rated How To Calculate Mole Ratio pictures upon internet. For each one mole of CuSO 4 there are 5 moles of water. The measure used to compare quantities of atoms is the mole. That means that answer choice a would be considered by most teachers to be the correct answer. These molar ratios can also be expressed as fractions.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate mole ratio of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






