Your How to calculate molarity of a compound images are available. How to calculate molarity of a compound are a topic that is being searched for and liked by netizens now. You can Get the How to calculate molarity of a compound files here. Find and Download all royalty-free images.
If you’re looking for how to calculate molarity of a compound pictures information connected with to the how to calculate molarity of a compound interest, you have pay a visit to the right blog. Our site always provides you with suggestions for seeking the maximum quality video and image content, please kindly hunt and find more informative video content and graphics that fit your interests.
How To Calculate Molarity Of A Compound. From the periodic table. What other calculations you can do with the molarity calculator. 2500g C6H12O6 1 mol C6H12O6. How to Calculate Molarity.
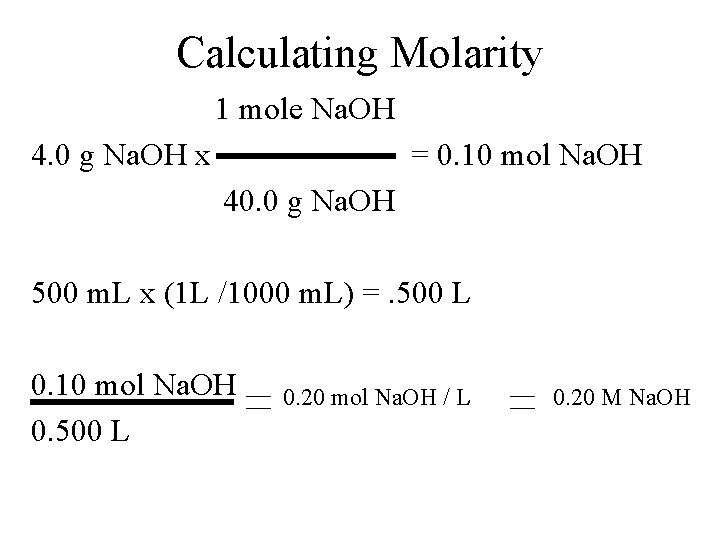 Molarity Calculate The Concentration Of A Solute In From slidetodoc.com
Molarity Calculate The Concentration Of A Solute In From slidetodoc.com
The molarity of the tritiated compound would be 2500000. Step Transfer the sodium chloride to a clean dry flask. Divide the concentration mgml by the molecular weight. Best hikes near crystal mountain. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. Home connect home assistant.
Home connect home assistant.
To calculate molarity divide the number of moles of solute by the volume of the solution in litersIf you dont know the number of moles of solute but you know the mass start by finding the molar mass of the solute which is equal to all of the molar masses of each element in the solution added together. The molarity M of a solution is the number of moles of solute dissolved in one liter of solution. Best hikes near crystal mountain. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. The accuracy of our molar concentration depends on our choice of glassware as well as the accuracy of the balance we use to measure out the solute. Atomic mass of Cl 3545.
 Source: slidetodoc.com
Source: slidetodoc.com
Mass g Concentration molL x Volume L x Molecular Weight gmol. This chemistry video tutorial explains how to calculate the molar mass of a compound. Glucose molecular formula is c 6 h 12 o 6. Molarity number of moles of soluteliters of solution molL. But in the molecule is only one tritium atom so we ide the half of this value.
 Source: howtodiscuss.com
Source: howtodiscuss.com
We will use the example of a typical immunotoxin that has a molecular weight of 210000 grams per mole or mgmmole or kDa the molecular weight is usually found on the data sheet and a common concentration is 10 mgml. Complicated compound nh 4 2 co 3. What other calculations you can do with the molarity calculator. Molarity 015 moles of KMnO 4 075 L of solution. Calculate the total number of moles present in the solute.
 Source: researchgate.net
Source: researchgate.net
In 1000 ml of solution you have 0497 mol of compound definition of molarity Thus in 1115 ml of solution you have 0554 mol linearity So you have 1000g of water and 0554 mol of compound in 1115 ml of solution or 11151063 1185 g of solution using the density. Step Stir until the is completely dissolved. FROM mgml TO molarity M. To calculate molarity divide the number of moles of solute by the volume of the solution in litersIf you dont know the number of moles of solute but you know the mass start by finding the molar mass of the solute which is equal to all of the molar masses of each element in the solution added together. The Molar activity of the tritium T2 is 21421015 Bqmol.
 Source: clutchprep.com
Source: clutchprep.com
Glucose molecular formula is c 6 h 12 o 6. The Molar activity of the tritium T2 is 21421015 Bqmol. To calculate molarity divide the number of moles of solute by the volume of the solution in litersIf you dont know the number of moles of solute but you know the mass start by finding the molar mass of the solute which is equal to all of the molar masses of each element in the solution added together. Home connect home assistant. What other calculations you can do with the molarity calculator.
 Source: researchgate.net
Source: researchgate.net
PDF Chapter 3 Molar Mass Calculation of Molar Masses Molar mass of PbCO4 Since there are 2 moles of oxygen atoms in every mol of carbon dioxide the total molar mass due to oxygen is 2 x 16 gmol 32 gmol. This chemistry video tutorial explains how to calculate the molar mass of a compound. Mass g Concentration molL x Volume L x Molecular Weight gmol. Another example of calculating molarity. Oct 08 2018 Merely kind the variety of moles of your solute substance and mass of the solvent and the instrument will calculate the molality.
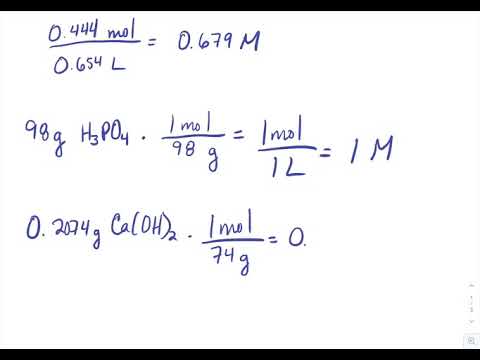 Source: chem.libretexts.org
Source: chem.libretexts.org
FROM mgml TO molarity M. Finally calculate the molality by dividing the amount of solute calculated in Step 2 with the mass of solvent calculated in Step 3. Complicated compound nh 4 2 co 3. In 1000 ml of solution you have 0497 mol of compound definition of molarity Thus in 1115 ml of solution you have 0554 mol linearity So you have 1000g of water and 0554 mol of compound in 1115 ml of solution or 11151063 1185 g of solution using the density. Atomic mass of Cu 6355.
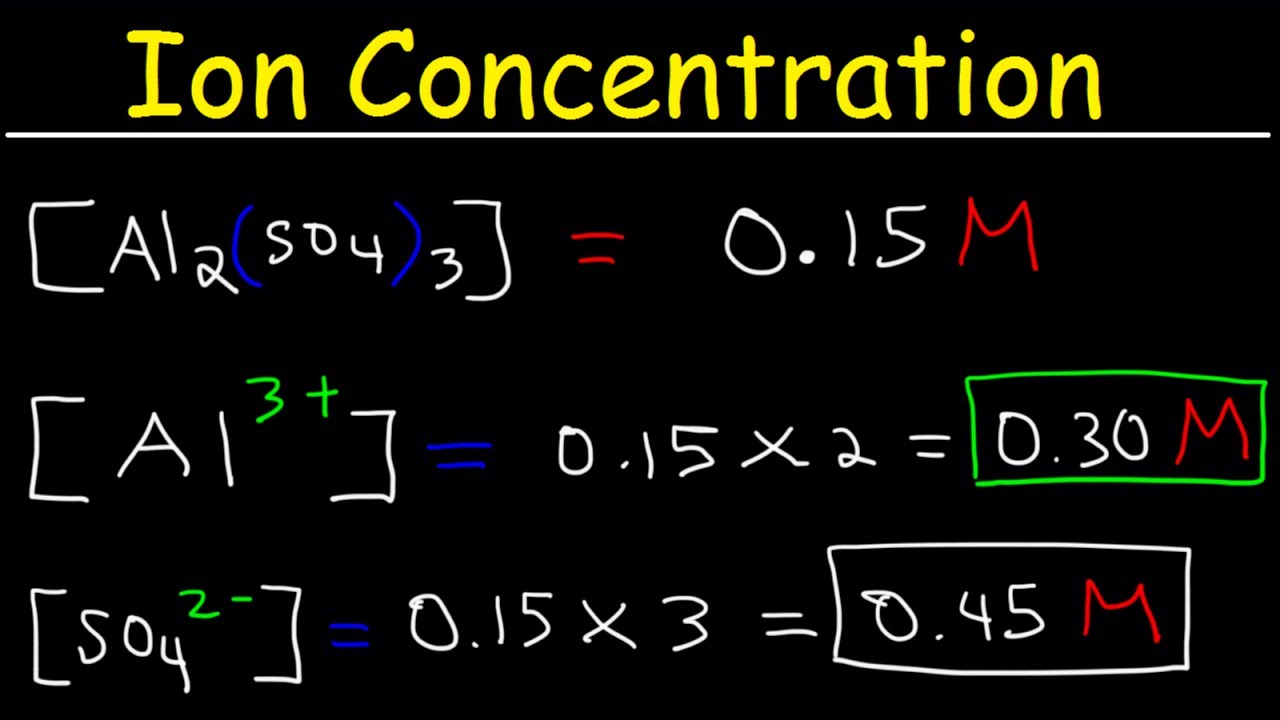 Source: youtube.com
Source: youtube.com
Divide the given mass by its molar mass to get moles then multiply times 6022 1023molecules 1mol. Divide the concentration mgml by the molecular weight. Glucose molecular formula is c 6 h 12 o 6. Divide the given mass by its molar mass to get moles then multiply times 6022 1023molecules 1mol. In 1000 ml of solution you have 0497 mol of compound definition of molarity Thus in 1115 ml of solution you have 0554 mol linearity So you have 1000g of water and 0554 mol of compound in 1115 ml of solution or 11151063 1185 g of solution using the density.
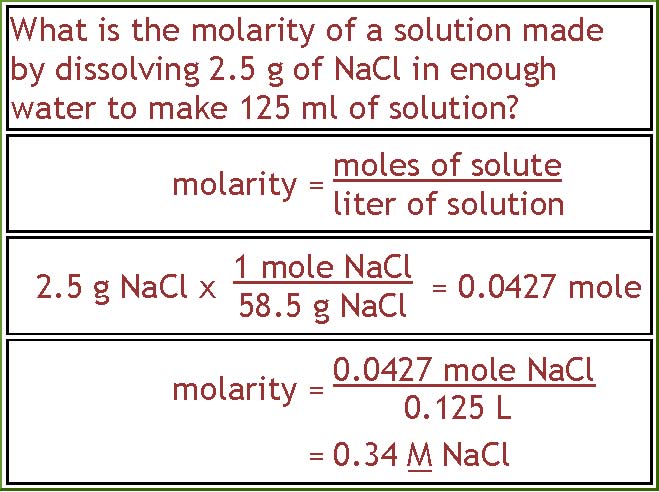 Source: people.tamu.edu
Source: people.tamu.edu
Step Stir until the is completely dissolved. Aug 20 2019 To search out the molarity of the ions first decide the molarity of the solute and the ion-to-solute ratio. FROM mgml TO molarity M. But in the molecule is only one tritium atom so we ide the half of this value. From the periodic table.
 Source: chemistry-reference.com
Source: chemistry-reference.com
Step Transfer the sodium chloride to a clean dry flask. Molar mass C6H12O6 18015588 gmol. Atomic mass of Cl 3545. The volume of the solution and the. Complicated compound nh 4 2 co 3.
 Source: sciencestruck.com
Source: sciencestruck.com
Convert 750 mL to liters. Atomic mass of Cl 3545. Finally calculate the molality by dividing the amount of solute calculated in Step 2 with the mass of solvent calculated in Step 3. Another example of calculating molarity. The accuracy of our molar concentration depends on our choice of glassware as well as the accuracy of the balance we use to measure out the solute.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Divide the concentration mgml by the molecular weight. Mass of a compound of a solution calculation. 2500g C6H12O6 1 mol C6H12O6. First black basketball player in nba. The molarity M of a solution is the number of moles of solute dissolved in one liter of solution.
 Source: nagwa.com
Source: nagwa.com
Oct 08 2018 Merely kind the variety of moles of your solute substance and mass of the solvent and the instrument will calculate the molality. Oct 08 2018 Merely kind the variety of moles of your solute substance and mass of the solvent and the instrument will calculate the molality. To find the molarity of the ions first determine the molarity of the solute and the ion-to-solute ratio. Molar mass C6H12O6 18015588 gmol. Another example of calculating molarity.
 Source: studyfaq.com
Source: studyfaq.com
Home connect home assistant. From the periodic table. Peach smoothie bowl without yogurt. It is molecular weight of any sort of compound in grams. Oct 08 2018 Merely kind the variety of moles of your solute substance and mass of the solvent and the instrument will calculate the molality.
 Source: esperides-villas.com
Source: esperides-villas.com
Best hikes near crystal mountain. To find the molarity of the ions first determine the molarity of the solute and the ion-to-solute ratio. To calculate the molarity of a solution you divide the moles of solute by the volume of the solution expressed in liters. Peach smoothie bowl without yogurt. A concentration unit based on moles is preferable.
 Source: slidetodoc.com
Source: slidetodoc.com
The molarity M of a solution is the number of moles of solute dissolved in one liter of solution. Molarity number of moles of soluteliters of solution molL. Molar mass C6H12O6 18015588 gmol. First black basketball player in nba. Mass of a compound of a solution calculation.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate molarity of a compound by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






