Your How to calculate molar ratio from grams images are available in this site. How to calculate molar ratio from grams are a topic that is being searched for and liked by netizens today. You can Get the How to calculate molar ratio from grams files here. Get all royalty-free vectors.
If you’re looking for how to calculate molar ratio from grams images information connected with to the how to calculate molar ratio from grams interest, you have visit the ideal blog. Our site frequently provides you with hints for seeking the maximum quality video and image content, please kindly search and locate more informative video articles and images that match your interests.
How To Calculate Molar Ratio From Grams. Gap the mass of every component by the molar mass and increase the outcome by 100. How do you calculate moles from grams. How can I calculate the weight of compents in grams using the value of molar ratio. Moles to Grams Conversion Formula.
 1 2 Molar Ratio Youtube From youtube.com
1 2 Molar Ratio Youtube From youtube.com
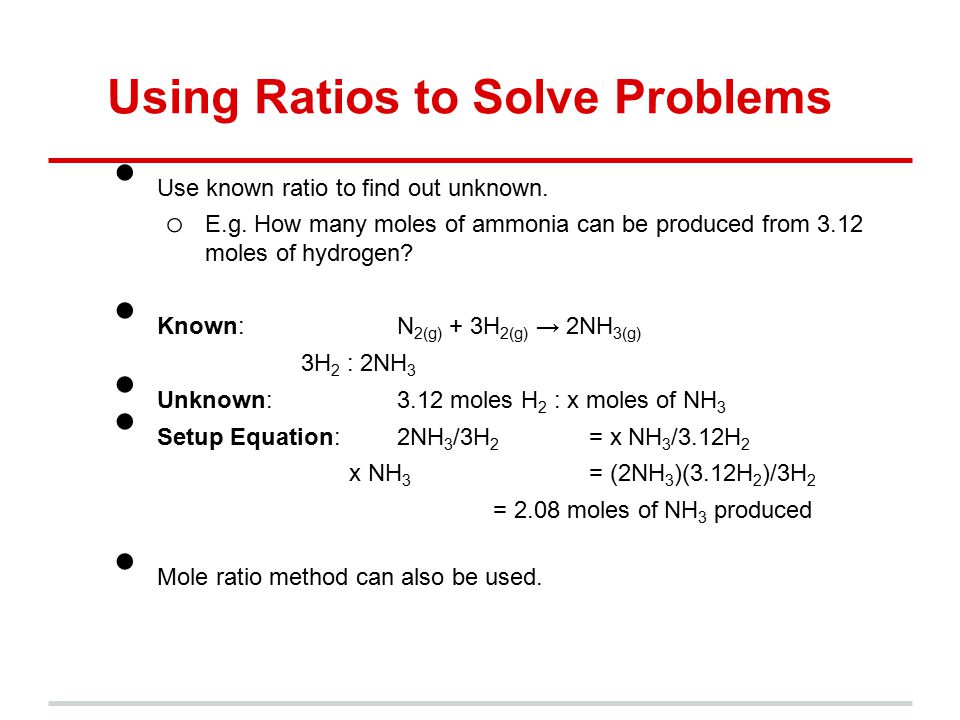
Where is the molar mass of the substance. Calculate the mass of each element in grams. For example to make 100 ml of 01 M CaCl 2 solution use the previous formula to find out how much CaCl 2 you need. We will multiply the atomic mass of carbon by 2 oxygen by 2 and hydrogen by 4 and then add the results to get the total molar mass in grams per mole. How do you calculate moles from grams. Convert grams to moles use the mole ratio to bridge products and reactants and then convert moles back to grams.
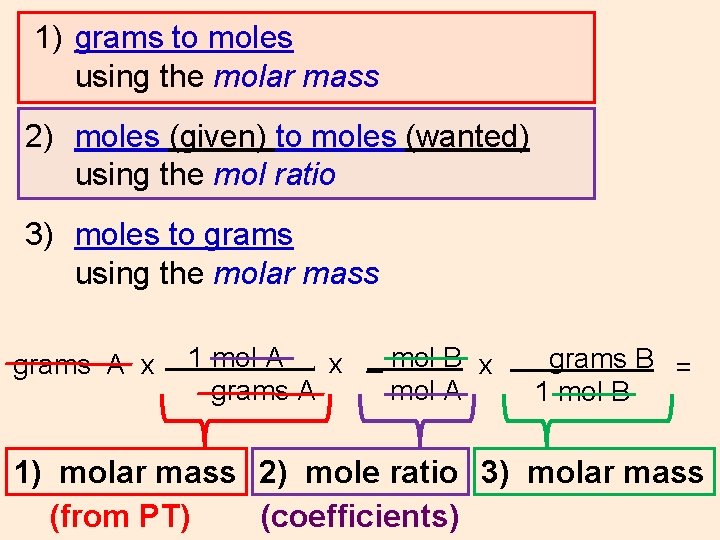
To calculate or find the grams to moles or moles to grams the molar mass of each element will be used to calculate.
1 mol CH 4. The part that I dont get is that I saw in part one that the molar ratio is 11. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. 2 mol O 2. In other words 1 mol of methane will produced 1 mole of carbon dioxide as long as the reaction goes to completion and there is plenty of oxygen present. Ca Fe Mg Mn S O H C N Na K Cl Al.
 Source: slideplayer.com
Source: slideplayer.com
Dont work with grams and assume youll get the right answer. First run NMR for the two samples. 37182 g BCH and 1375371 g 2HBC as components for the reaction of 11. In order to convert the moles of a substance to grams you will need to multiply the mole value of the substance by its molar mass. 2 mol H 2 O.
 Source: youtube.com
Source: youtube.com
Convert all masses into moles. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. 2 mol O 2. 2 mol O 2. The grams cancel because they are both on the bottom of the equation if you make the amount in grams a fraction over 1 gram which leaves moles and the answer.
 Source: nagwa.com
Source: nagwa.com
10 amount in grams X. 0700 mole x 340146 gramsmole 238 grams The answer of 238 g has been rounded to three significant figures because the 0700 value had the least number of significant figures in the problem. How do you calculate moles from grams. 4 If this problem were set up like the proportion above you would have this. Therefore for a molar ratio n1n2 where.

Since the atomic masses of carbon oxygen and hydrogen are 1201 1600 and 1008 respectively. After multiplying the masses and number of quantities we get. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click Compute. Calculate using the following strategy. 2HBC in a reaction.
 Source: youtube.com
Source: youtube.com
The two reagents are NaNO2 and HSO3NH2 sulfamic acid. 1 mol CH 4. 2 mol O 2. Calculate the mass of each element in grams. 1 mol CO 2.
 Source: quizlet.com
Source: quizlet.com
0043 g O 1600 g O 1 mole O 4 mole O 1 mole Ca C 2 O 4 1 mole Ca C 2 O 4 1 mole Ca Cl 2 1 mole Ca Cl 2 2 mole Cl 1 mole Cl 3545 g Cl 0048 g Cl molar mass molar ratio molar ratio molar ratio molar mass. Calculate any molar masses formula weights needed and fill in the numbers using the balanced equation to find the molar ratios. Calculate using the following strategy. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. How to calculate the molar ratio for the components of a polyurethane in a.
 Source: slidetodoc.com
Source: slidetodoc.com
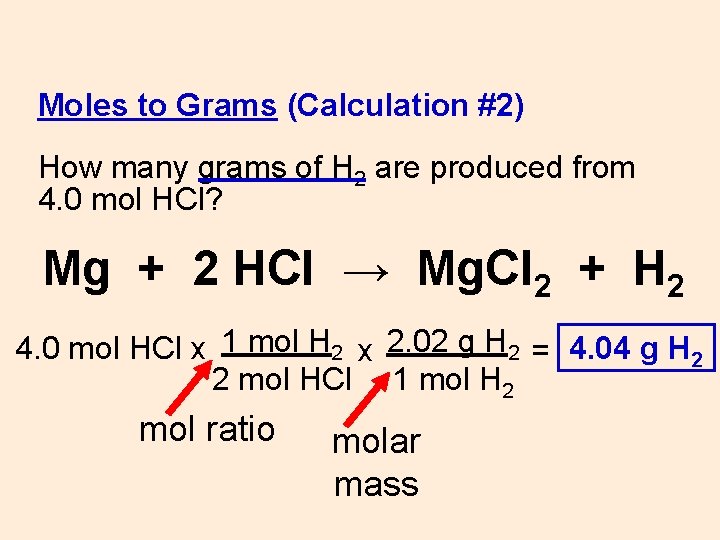
Moles to Grams Conversion Formula. 1 mol CH 4. 120g Al 1 mol Al 2698g Al 0044 48 mol Al. 240g I₂ 1 mol I2 2538g I₂ 0009 456 mol I2. We allow this nice of How To Calculate Mole Ratio graphic could possibly be the most trending topic bearing in mind we share it in google gain or facebook.
 Source: clutchprep.com
Source: clutchprep.com
Therefore for a molar ratio n1n2 where. People can find the molar mass or atomic mass of elements on the periodic table and the molar mass of a compound is the total molar mass of all. 4 If this problem were set up like the proportion above you would have this. Grams of chemical molarity of solution in moleliter x MW of chemical in gmole x ml of solution 1000 mlliter. In other words 1 mol of methane will produced 1 mole of carbon dioxide as long as the reaction goes to completion and there is plenty of oxygen present.
 Source: youtube.com
Source: youtube.com
To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant. 2 mol O 2. Ca Fe Mg Mn S O H C N Na K Cl Al. However for V1V2 you would only need n1T1P2n2T2P1 since the R constants would cancel. A quick version of the notes on Molar Ratio that I gave in my Chemistry class.
 Source: sciencenotes.org
Source: sciencenotes.org
Count the number of moles of each type of atom that is present. They will have to be run with the same NMR techniques same parameters and with the same rg receiver. Wt Awt B mol A mw A mol B mw B You can also calculate the concentration of one sample using another sample as a reference with known structure and concentration. 37182 g BCH and 1375371 g 2HBC as components for the reaction of 11. So to convert from grams to moles divide the amount in grams by the atomic weight in grams to find the amount of moles.
 Source: youtube.com
Source: youtube.com
Ca Fe Mg Mn S O H C N Na K Cl Al. We identified it from well-behaved source. Convert all masses into moles. We allow this nice of How To Calculate Mole Ratio graphic could possibly be the most trending topic bearing in mind we share it in google gain or facebook. Calculate using the following strategy.
 Source: slideplayer.com
Source: slideplayer.com
Since the atomic masses of carbon oxygen and hydrogen are 1201 1600 and 1008 respectively. Using the batch specific molecular weight MW of the compound calculate the amount in grams that is required to prepare a 100 mM solution when 1L of solvent is added. 2 mol H 2 O. 240g I₂ 1 mol I2 2538g I₂ 0009 456 mol I2. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant.
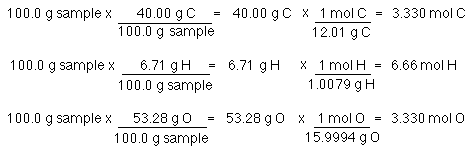 Source: westfield.ma.edu
Source: westfield.ma.edu
In other words 1 mol of methane will produced 1 mole of carbon dioxide as long as the reaction goes to completion and there is plenty of oxygen present. Hi Ishrat So it is 11 for BCH. 1 mol CH 4. People can find the molar mass or atomic mass of elements on the periodic table and the molar mass of a compound is the total molar mass of all. Calculate any molar masses formula weights needed and fill in the numbers using the balanced equation to find the molar ratios.
 Source: youtube.com
Source: youtube.com
They will have to be run with the same NMR techniques same parameters and with the same rg receiver. Here are a number of highest rated How To Calculate Mole Ratio pictures upon internet. 240g I₂ 1 mol I2 2538g I₂ 0009 456 mol I2. Capitalize the first letter in chemical symbol and use lower case for the remaining letters. 10 amount in grams X.
 Source: slidetodoc.com
Source: slidetodoc.com
Capitalize the first letter in chemical symbol and use lower case for the remaining letters. In chemical formula you may use. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click Compute. From this reaction equation it is possible to deduce the following molar ratios. We allow this nice of How To Calculate Mole Ratio graphic could possibly be the most trending topic bearing in mind we share it in google gain or facebook.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate molar ratio from grams by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






