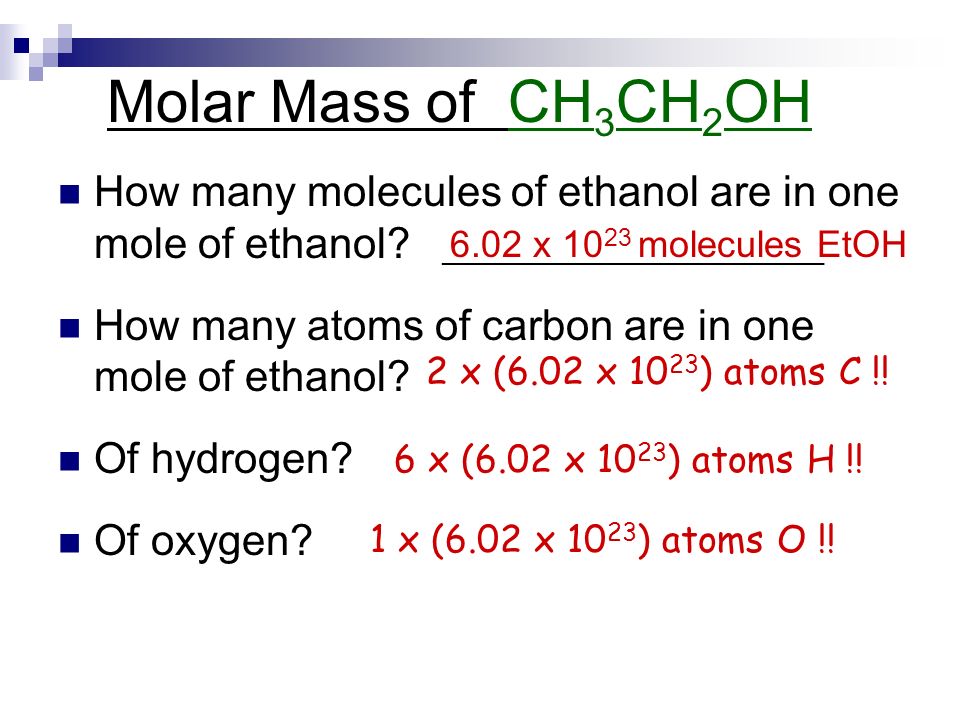
Your How to calculate molar mass with moles images are ready in this website. How to calculate molar mass with moles are a topic that is being searched for and liked by netizens today. You can Find and Download the How to calculate molar mass with moles files here. Find and Download all free vectors.
If you’re looking for how to calculate molar mass with moles pictures information linked to the how to calculate molar mass with moles keyword, you have visit the ideal site. Our website always provides you with hints for viewing the maximum quality video and picture content, please kindly surf and locate more enlightening video articles and images that fit your interests.
How To Calculate Molar Mass With Moles. Therefore NaCl 584538 gL. Finding molar mass also called molecular weight molecular mass and gram formula mass is an essential skill in chemistry especially for mole to gram conv. Mass g molar mass g mol -1 moles mol From the data in the table and its graphical representation we can generalise and say that for any pure substance the mass of substance in grams is equal to the moles of substance multiplied by the mass of 1 mole of the substance. Write the equation for calculating molality.
 Formula Triangle Molarity Teaching Chemistry Chemistry Lessons Chemistry Foldables From pinterest.com
Formula Triangle Molarity Teaching Chemistry Chemistry Lessons Chemistry Foldables From pinterest.com
NaCl Na Cl. M m n. Where n is the number of moles and V is the volume in litres. This is defined as 0001 kilogram per mole or 1 gram per mole. Molar Mass Mass of the Substance KgAmount of Substance Mol Mole or mol is the unit used to measure the amount of a substance. First of all before you can use this equation you need to know how many moles of solute are there in the solution.
The molar mass of nacl is 5844 gmol.
Molality molessolute masssolvent in kg Identify the solute and solvent that make up the solution. The mole the unit of measurement for amount of substance is defined in such a way that the molar mass of a compound in gmol is numerically equal to the average mass of one molecule in. Find the molar mass of a substance with a mass of 14 kg and number of moles of 28 mol. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. Molar Mass 1000 1574.
 Source: pinterest.com
Source: pinterest.com
Determine the molar concentration of the unknown in the solution from the observed osmotic pressure. In chemistry the molar mass of a chemical compound is defined as the mass of 1 mole or 60221410 23 particles of the substance expressed in grams. This is defined as 0001 kilogram per mole or 1 gram per mole. All you need to do is find the atomic mass of the element on the periodic table and report the number with the unit grams per mole or gmol. Mass g molar mass g mol -1 moles mol From the data in the table and its graphical representation we can generalise and say that for any pure substance the mass of substance in grams is equal to the moles of substance multiplied by the mass of 1 mole of the substance.
 Source: pinterest.com
Source: pinterest.com
M Mass n Number of moles M Molar MassLets solve. M Mass n Number of moles M Molar MassLets solve. From this you can see that sodiums molar mass will be 2299 gmol. How to convert mass to mole. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl.
 Source: pinterest.com
Source: pinterest.com
NaCl Na Cl. Where m is the mass n the number of moles and M the resulting molar mass. Molecules and atoms are very tiny both in size and mass. First of all before you can use this equation you need to know how many moles of solute are there in the solution. This mole conversion calculator also helps you calculate molar mass of a substance using a similar mathematical approach but in less time.
 Source: pinterest.com
Source: pinterest.com
To compute for the molar mass two essential parameters are needed and these parameters are mass m and number of moles n. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. How to calculate molar mass. The formula for calculating the molar mass. Determine the molar concentration of the unknown in the solution from the observed osmotic pressure.
 Source: pinterest.com
Source: pinterest.com
Multiply the atomic weight of each element with its number of atoms present in the compound. Number of moles mass relative formula mass This can be rearranged to find the mass if the number of moles and molar mass its relative formula mass in grams are known. Number of Moles M a s s Molar Mass. Molar Mass 1000 1574. Convert grams kc4h5o6 to moles or moles kc4h5o6 to grams.
 Source: pinterest.com
Source: pinterest.com
We all want to know that in a particular substance how many molecules are present. Mass to Mole Calculator - ezcalcme tip ezcalcme. Lets solve an example. Mass g molar mass g mol -1 moles mol From the data in the table and its graphical representation we can generalise and say that for any pure substance the mass of substance in grams is equal to the moles of substance multiplied by the mass of 1 mole of the substance. The molar mass is calculated by measuring the mass and the number of moles in a sample of the substance of interest and then dividing both amounts.
 Source: pinterest.com
Source: pinterest.com
The molar mass is the weight of one sample mole. Determine the molar concentration of the unknown in the solution from the observed osmotic pressure. The molar mass is the weight of one sample mole. Mass to Mole Calculator - ezcalcme tip ezcalcme. This is defined as 0001 kilogram per mole or 1 gram per mole.
 Source: pinterest.com
Source: pinterest.com
How to convert mass to mole. Determine the moles of unknown the solute from the molarity of the solution and the volume in liters of the solution. To compute for the molar mass two essential parameters are needed and these parameters are mass m and number of moles n. Write the equation for calculating molality. This chemistry video tutorial explains how to calculate the molar mass of a compound.
 Source: pinterest.com
Source: pinterest.com
Write the units based on molar mass information and set up fractions to correctly cancel the units. Lets solve an example. How to convert mass to mole. How do you calculate moles from molarity. Molality molessolute masssolvent in kg Identify the solute and solvent that make up the solution.
 Source: pinterest.com
Source: pinterest.com
Write the equation for calculating molality. Determine the moles of unknown the solute from the molarity of the solution and the volume in liters of the solution. For example the atomic mass of titanium is 4788 amu or 4788 gmol. Number of Moles M a s s Molar Mass. Convert grams kc4h5o6 to moles or moles kc4h5o6 to grams.
 Source: pinterest.com
Source: pinterest.com
This mole conversion calculator also helps you calculate molar mass of a substance using a similar mathematical approach but in less time. Molar Mass Mass of the Substance KgAmount of Substance Mol Mole or mol is the unit used to measure the amount of a substance. Molecules and atoms are very tiny both in size and mass. All you need to do is find the atomic mass of the element on the periodic table and report the number with the unit grams per mole or gmol. Determine the moles of unknown the solute from the molarity of the solution and the volume in liters of the solution.
 Source: pinterest.com
Source: pinterest.com
Add up all and assign unit as gramsmole. Make use of the chemical formula to determine the number of atoms of each element in the compound. This chemistry video tutorial explains how to calculate the molar mass of a compound. For example the atomic mass of titanium is 4788 amu or 4788 gmol. Multiply the atomic weight of each element with its number of atoms present in the compound.
 Source: pinterest.com
Source: pinterest.com
How to Calculate and solve for the Mass Number of Moles. Multiply moles by the molar mass to convert from moles to grams and divide grams by the molar mass to convert from grams to moles. Where m is the mass n the number of moles and M the resulting molar mass. Add up all and assign unit as gramsmole. This converts atomic units to grams per mole.
 Source: pinterest.com
Source: pinterest.com
For example the atomic mass of titanium is 4788 amu or 4788 gmol. Add up all and assign unit as gramsmole. Make use of the chemical formula to determine the number of atoms of each element in the compound. Determine the molar mass from the mass of the unknown and the number of moles of unknown. M Molar mass m Mass n Number of moles.
 Source: pinterest.com
Source: pinterest.com
Determine the molar concentration of the unknown in the solution from the observed osmotic pressure. How do you calculate moles from molarity. Determine the molar concentration of the unknown in the solution from the observed osmotic pressure. The molar mass is calculated by measuring the mass and the number of moles in a sample of the substance of interest and then dividing both amounts. Where m is the mass n the number of moles and M the resulting molar mass.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate molar mass with moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.