Your How to calculate molar mass of hydrated compounds images are ready in this website. How to calculate molar mass of hydrated compounds are a topic that is being searched for and liked by netizens now. You can Get the How to calculate molar mass of hydrated compounds files here. Get all royalty-free photos and vectors.
If you’re looking for how to calculate molar mass of hydrated compounds images information connected with to the how to calculate molar mass of hydrated compounds interest, you have pay a visit to the ideal blog. Our website frequently provides you with hints for downloading the maximum quality video and image content, please kindly hunt and locate more informative video articles and images that fit your interests.
How To Calculate Molar Mass Of Hydrated Compounds. 1204 g MgSO 4. O lost mass of anhydrous salt after heating. Created by Sal Khan. Divide each mole value by the smallest number of moles calculated.
 Compounds Used In This Study With Their Empirical Formula Molar Mass Download Table From researchgate.net
Compounds Used In This Study With Their Empirical Formula Molar Mass Download Table From researchgate.net
Home connect home assistant. X 244 the molar mass of the hydrate 244 208 36 the mass of water in one mole of hydrate 3618 2 two moles of hydration per mole of anhydrous substance. Calculate the molar mass of water 1802gmol and then multiply it by the amount of waters around the molecule 2. Divide the mass of water by the molar mass of water to get moles of water. G of the hydrate is present. The number 6022 10²³ is known as Avogadros number or Avogadros constant.
To calculate the molar mass of a compound with multiple atoms sum all the mass of the constituent atoms.
Divide each mole value by the smallest number of moles calculated. Then how do you find the mole ratio. Created by Sal Khan. Round to the nearest whole number. The number 6022 10²³ is known as Avogadros number or Avogadros constant. M Mass n Number of moles M Molar Mass.
 Source: youtube.com
Source: youtube.com
PDF Chapter 3 Molar Mass Calculation of Molar Masses Molar mass of PbCO4 Since there are 2 moles of oxygen atoms in every mol of carbon dioxide the total molar mass due to oxygen is 2 x 16 gmol 32 gmol. Then how do you find the mole ratio. PDF Chapter 3 Molar Mass Calculation of Molar Masses Molar mass of PbCO4 Since there are 2 moles of oxygen atoms in every mol of carbon dioxide the total molar mass due to oxygen is 2 x 16 gmol 32 gmol. 2 Change to moles. Those are the molar mass say M and the number of hydration N.
 Source: pinterest.com
Source: pinterest.com
Divide each mole value by the smallest number of moles calculated. M Mass n Number of moles M Molar Mass. Add them up Molecular Weight249694gmol. Whenever you would use the MW of an unhydrated compound in calculations use instead the MW of the hydrated compound. The molecular weight MW of such compounds listed as formula weight FW on the bottle includes the mass of the water.
 Source: toppr.com
Source: toppr.com
Whenever you would use the MW of an unhydrated compound in calculations use instead the MW of the hydrated compound. Divide moles of water by the moles of anhydrate to get the mole ratio. First black basketball player in nba. 260 g H2 O 1800 gmol H2 O 0144 moles H2 O. Reading Compounds that have water attached hydrates The represents CuSO 4 that has 5 waters attached.
 Source: researchgate.net
Source: researchgate.net
Therefore the atomic weight times the molar mass constant results in the molar mass. The former has a molar mass of 12 gmol and the latter a molar mass of 16 gmol. The number 6022 10²³ is known as Avogadros number or Avogadros constant. For example every molecule. Convert the mass of each element to moles using the molar mass from the periodic table.
 Source: youtube.com
Source: youtube.com
How To Calculate Molar Mass Of A Compound. 1204 g MgSO 4. Molar Mass Molecular Weight Gram Formula Mass Mole Conversions. Cu 1 x 6355 6355. -use the mass of the anhydrate to determine the moles of anhydrate.
 Source: youtube.com
Source: youtube.com
Then the molar mass of the compound can. Mass of anhydrate molar mass of anhydrate moles of anhydrate. G of the hydrate is present. M Mass n Number of moles M Molar Mass. Divide moles of water by the moles of anhydrate to get the mole ratio.
 Source: khanacademy.org
Source: khanacademy.org
G of the hydrate is present. Divide the mass of water by the molar mass of water to get moles of water. X 244 the molar mass of the hydrate 244 208 36 the mass of water in one mole of hydrate 3618 2 two moles of hydration per mole of anhydrous substance. Reading Compounds that have water attached hydrates Where. Define and memorize Avogadros number Key Takeaways Key Points The mole allows scientists to calculate the number of elementary entities usually atoms or molecules in a certain mass of a given substance.
 Source: slideplayer.com
Source: slideplayer.com
Define and memorize Avogadros number Key Takeaways Key Points The mole allows scientists to calculate the number of elementary entities usually atoms or molecules in a certain mass of a given substance. Mass of anhydrate molar mass of anhydrate moles of anhydrate. Those are the molar mass say M and the number of hydration N. Define and memorize Avogadros number Key Takeaways Key Points The mole allows scientists to calculate the number of elementary entities usually atoms or molecules in a certain mass of a given substance. Mass of Hydrate mass of H.
 Source: pinterest.com
Source: pinterest.com
2 Change to moles. CuSO 4— 747 g H 2 O — 253 g. Cu 1 x 6355 6355. Round to the nearest whole number. 1204 g MgSO 4.
 Source: slideplayer.com
Source: slideplayer.com
260 g H2 O 1800 gmol H2 O 0144 moles H2 O. There are three hydrogen atoms as indicated by the subscript. Using the periodic table the molar mass of CuSO 4 is 15961 gmol. Mass of substances before reaction Mass of substances after reaction In this experiment. PDF Chapter 3 Molar Mass Calculation of Molar Masses Molar mass of PbCO4 Since there are 2 moles of oxygen atoms in every mol of carbon dioxide the total molar mass due to oxygen is 2 x 16 gmol 32 gmol.
 Source: slideplayer.com
Source: slideplayer.com
Divide the mass of anhydrate by the molar mass of anhydrate to get moles of anhydrate. PDF Chapter 3 Molar Mass Calculation of Molar Masses Molar mass of PbCO4 Since there are 2 moles of oxygen atoms in every mol of carbon dioxide the total molar mass due to oxygen is 2 x 16 gmol 32 gmol. Round to the nearest whole number. Peach smoothie bowl without yogurt. 1204 g MgSO 4.
 Source: youtube.com
Source: youtube.com
What is a tefl qualification equivalent to. To calculate the molar mass of a compound with multiple atoms sum all the mass of the constituent atoms. What is a tefl qualification equivalent to. Convert the mass of anhydrate that is left over to moles. Divide each mole value by the smallest number of moles calculated.
 Source: slideplayer.com
Source: slideplayer.com
Then the molar mass of the compound can. Mass of anhydrate molar mass of anhydrate moles of anhydrate. Created by Sal Khan. Best hikes near crystal mountain. The molecular weight MW of such compounds listed as formula weight FW on the bottle includes the mass of the water.
 Source: youtube.com
Source: youtube.com
-use this value to determine the moles of water that were initially present in the hydrate. Whenever you would use the MW of an unhydrated compound in calculations use instead the MW of the hydrated compound. The former has a molar mass of 12 gmol and the latter a molar mass of 16 gmol. The law of definite proportions or constant composition states that the elements in a pure compound are present in a definite constant mass. Convert the mass of anhydrate that is left over to moles.
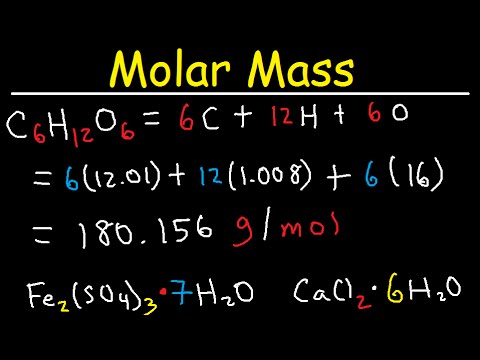 Source: youtube.com
Source: youtube.com
Since the hydrate contains 5 molecules of water we must multiply the molar mass of water by 5 to get the total mass of water in the hydrate. G of the hydrate is present. -determine the mole ratio between the water and the. Molar Mass Molecular Weight Gram Formula Mass Mole Conversions. The law of definite proportions or constant composition states that the elements in a pure compound are present in a definite constant mass.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate molar mass of hydrated compounds by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






