Your How to calculate molar mass in grams per mole images are ready in this website. How to calculate molar mass in grams per mole are a topic that is being searched for and liked by netizens now. You can Find and Download the How to calculate molar mass in grams per mole files here. Download all free photos and vectors.
If you’re searching for how to calculate molar mass in grams per mole images information connected with to the how to calculate molar mass in grams per mole keyword, you have come to the ideal blog. Our site always provides you with suggestions for downloading the highest quality video and image content, please kindly surf and locate more enlightening video content and graphics that match your interests.
How To Calculate Molar Mass In Grams Per Mole. Calculate the mass of 158 moles ch4 molar mass ch4 160 gmol given. First you must calculate the number of moles in this solution by rearranging the equation. Calculate the mass of a 2 moles and b 025 moles of iron. The answer should be rounded off to three significant figures resulting in 306 g.
 Determine The Mass Of Each Of The Followin Clutch Prep From clutchprep.com
Determine The Mass Of Each Of The Followin Clutch Prep From clutchprep.com
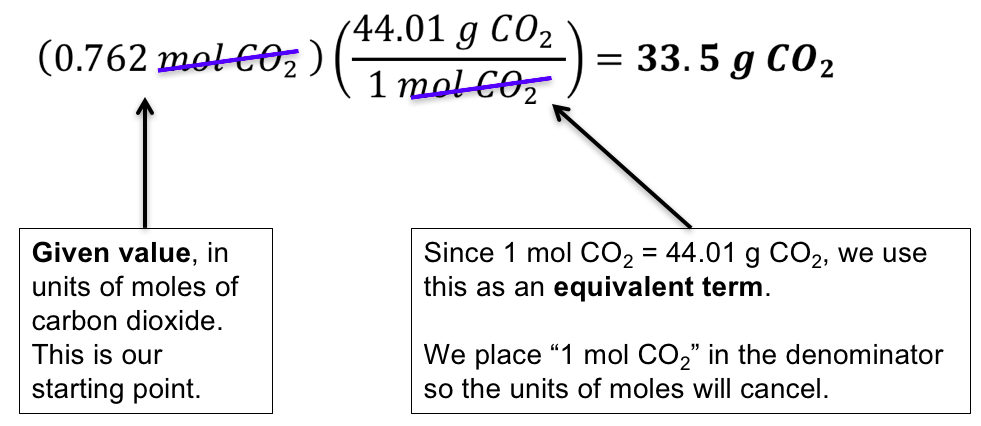
Once we get the value for moles we can then divide the mass of gas by moles to get the molar mass. Gram per mole gmol 1. Lets figure out what the difference between molar mass and atomic mass is and learn to use molar mass as a conversion factor and stop guessing on how to con. Since there are 2 moles of oxygen atoms in every mol of carbon dioxide the total molar mass due to oxygen is 2 x 16 gmol 32 gmol. Add up all. Mol H2O in 283 g H2O 283 g 18 gmol 1572 mol.
The formula for moles to grams is given by.
We can use the above equation to find the mass of a substance when we are given the number of moles of the substance. How many grams of KF molar mass581 gmole must be added to 283 grams of water molar mass180 gmole to make an aqueous solution that has a mole fraction of KF of 00855. The unit is typically gmol. N m M where M is the molar mass of this material. How do you convert molarity to grams per mole. The unit used to measure is grams per mole.
 Source: youtube.com
Source: youtube.com
The answer should be rounded off to three significant figures resulting in 306 g. This leads to two important facts. Therefore the mole is a unit for that physical quantity. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. The number of moles represents the fraction.
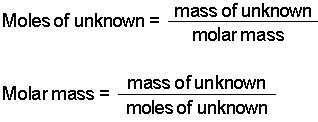 Source: chem.purdue.edu
Source: chem.purdue.edu
To correctly estimate the number of moles n of a substance of a specific mass m in grams you need to follow the grams to moles formula. The number of moles represents the fraction. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da. NaCl Na Cl. The formula for moles to grams is given by.
 Source: chemistrygod.com
Source: chemistrygod.com
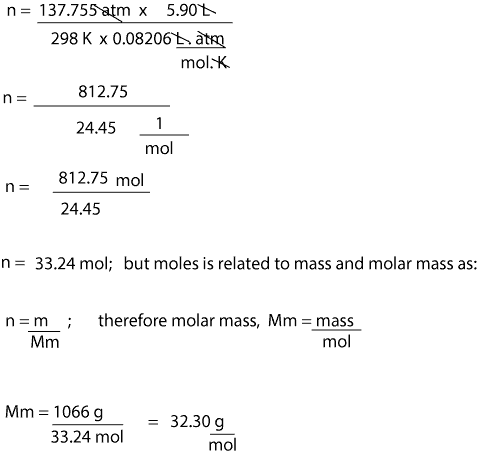
Recall that the ideal gas equation is given as. How many grams of KF molar mass581 gmole must be added to 283 grams of water molar mass180 gmole to make an aqueous solution that has a mole fraction of KF of 00855. Once we get the value for moles we can then divide the mass of gas by moles to get the molar mass. This leads to two important facts. Look for the atomic masses of hydrogen sulfur and oxygen.
 Source: inchcalculator.com
Source: inchcalculator.com
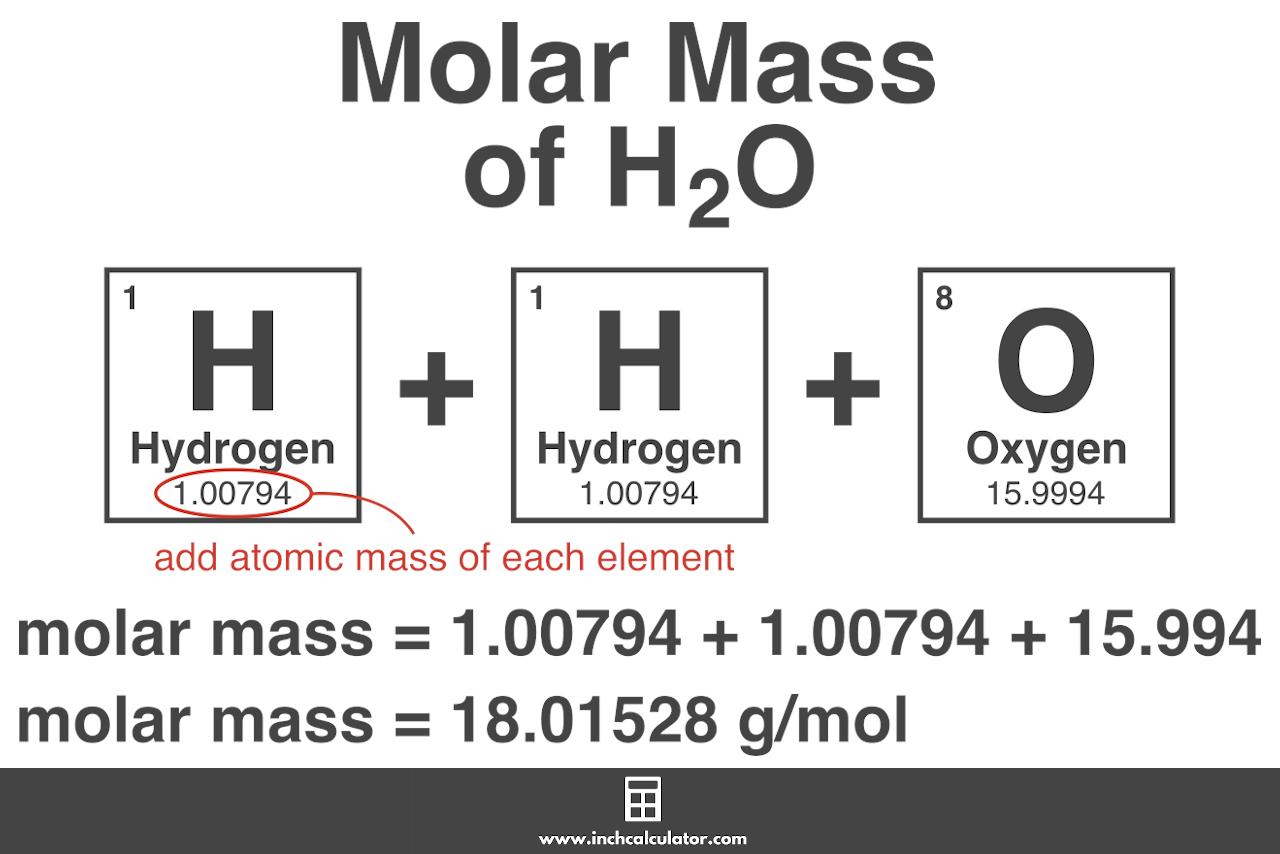
Calculate the mass of a 2 moles and b 025 moles of iron. Once we get the value for moles we can then divide the mass of gas by moles to get the molar mass. Mass g molar mass g mol -1 moles mol From the data in the table and its graphical representation we can generalise and say that for any pure substance the mass of substance in grams is equal to the moles of substance multiplied by the mass of 1 mole of the substance. You can use parenthesis or. NaCl Na Cl.
 Source: conquerchemistry.com
Source: conquerchemistry.com
NaCl 229898 gL 354530 gL. Multiply the atomic weight of each element with its number of atoms present in the compound. How many grams of KF molar mass581 gmole must be added to 283 grams of water molar mass180 gmole to make an aqueous solution that has a mole fraction of KF of 00855. 250 moles x 122550 gmole 306375 grams. Add up all.
 Source: wisc.pb.unizin.org
Source: wisc.pb.unizin.org
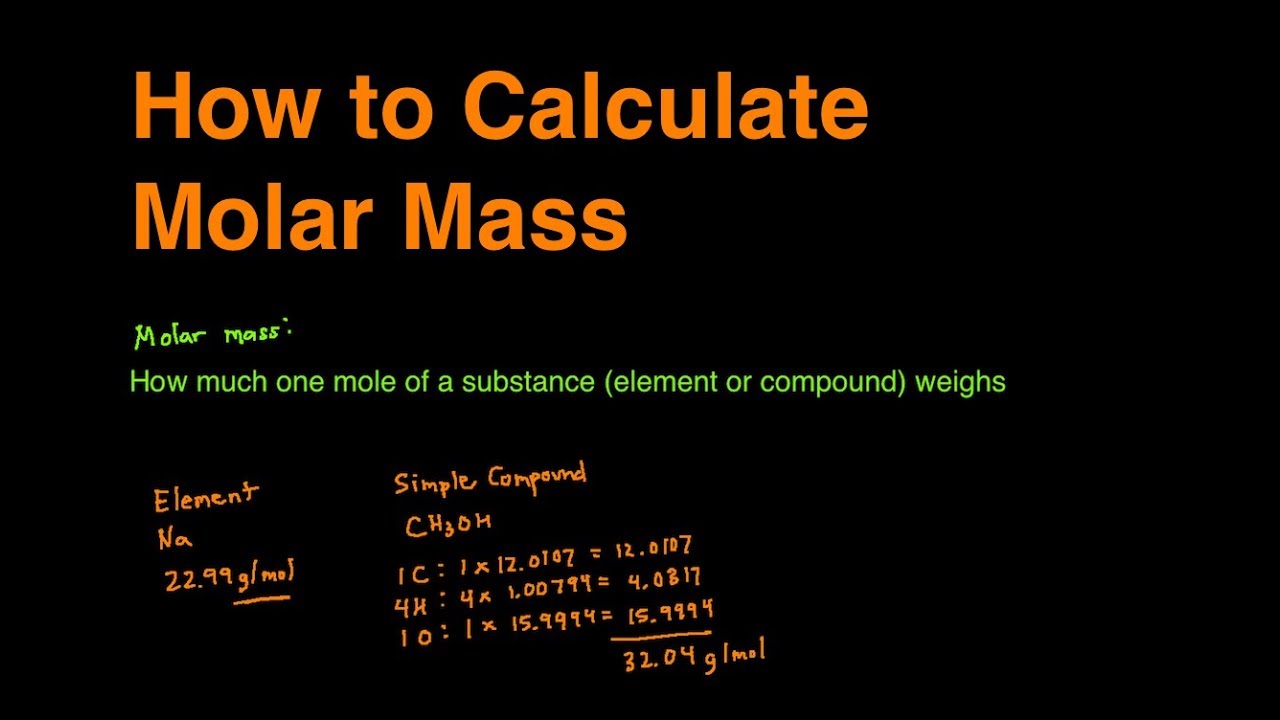
Mass g molar mass g mol -1 moles mol From the data in the table and its graphical representation we can generalise and say that for any pure substance the mass of substance in grams is equal to the moles of substance multiplied by the mass of 1 mole of the substance. The answer should be rounded off to three significant figures resulting in 306 g. Mass g No. Example If you want to find the molar mass of common salt Sodium Chloride- NaCl You add the mass of each element of it. Mass moles mass of 1 mole.
 Source: youtube.com
Source: youtube.com
How do you convert molarity to grams per mole. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. The resulting molar mass of carbon dioxide is therefore 12 gmol 32 gmol 44 gmol. Now we can use the rearranged equation. The answer should be rounded off to three significant figures resulting in 306 g.
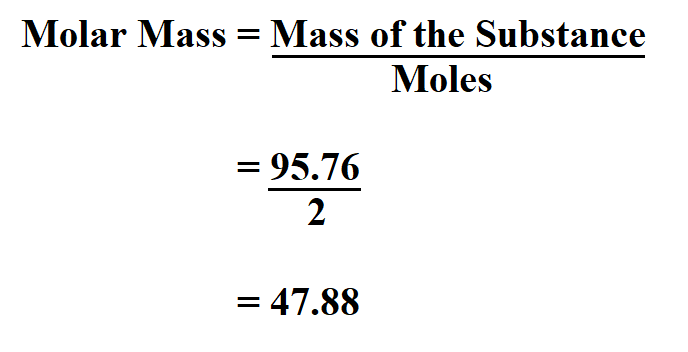 Source: learntocalculate.com
Source: learntocalculate.com
All you need to do is find the atomic mass of the element on the periodic table and report the number with the unit grams per mole or gmol. Multiply the atomic weight of each element with its number of atoms present in the compound. The formula for moles to grams is given by. Finding the molar mass of a single element is really simple. The unit is typically gmol.

Mass number of moles molar mass where mass is in grams and the molar mass is in grams per mole. N m M where M is the molar mass of this material. Molar mass massmole gmol. Now we can use the rearranged equation. Finding the molar mass of a single element is really simple.
 Source: khanacademy.org
Source: khanacademy.org
The unit is typically gmol. First you must calculate the number of moles in this solution by rearranging the equation. You can use parenthesis or. The former has a molar mass of 12 gmol and the latter a molar mass of 16 gmol. Calculate the mass of a 2 moles and b 025 moles of iron.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
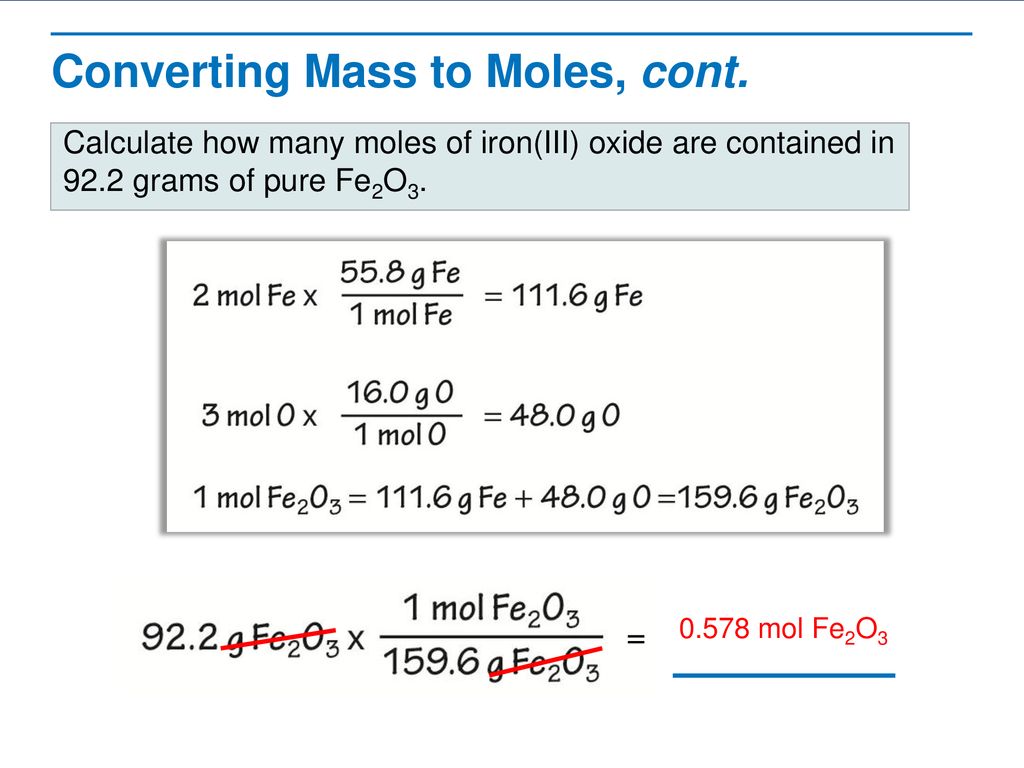
3 Following step three we obtain. How many grams of KF molar mass581 gmole must be added to 283 grams of water molar mass180 gmole to make an aqueous solution that has a mole fraction of KF of 00855. Mol H2O in 283 g H2O 283 g 18 gmol 1572 mol. Since there are 2 moles of oxygen atoms in every mol of carbon dioxide the total molar mass due to oxygen is 2 x 16 gmol 32 gmol. The molar mass also known as molecular weight is the sum of the total mass in grams of all the atoms that make up a mole of a particular molecule.
 Source: slideplayer.com
Source: slideplayer.com
All you need to do is find the atomic mass of the element on the periodic table and report the number with the unit grams per mole or gmol. How many grams of KF molar mass581 gmole must be added to 283 grams of water molar mass180 gmole to make an aqueous solution that has a mole fraction of KF of 00855. Mass g molar mass g mol -1 moles mol From the data in the table and its graphical representation we can generalise and say that for any pure substance the mass of substance in grams is equal to the moles of substance multiplied by the mass of 1 mole of the substance. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. You can use parenthesis or.
 Source: clutchprep.com
Source: clutchprep.com
Look for the atomic masses of hydrogen sulfur and oxygen. The unit used to measure is grams per mole. We can rearrange this equation in terms of moles n and then solve for its value. If we know mass pressure volume and temperature of a gas we can calculate its molar mass by using the ideal gas equation. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da.
 Source: slideserve.com
Source: slideserve.com
2 1008 3206 4 18 106076. The mass of one mole of carbon. All you need to do is find the atomic mass of the element on the periodic table and report the number with the unit grams per mole or gmol. Multiply the atomic weight of each element with its number of atoms present in the compound. U or Da is defined as 112 of the mass of the 12 C atom it follows that the molar mass of a substance measured in grams per mole is numerically equal to its mean atomic or molecular mass measured in Da.
 Source: clutchprep.com
Source: clutchprep.com
First you must calculate the number of moles in this solution by rearranging the equation. Multiply the atomic weight of each element with its number of atoms present in the compound. How many grams of KF molar mass581 gmole must be added to 283 grams of water molar mass180 gmole to make an aqueous solution that has a mole fraction of KF of 00855. How do you convert molarity to grams per mole. Use my code PRKU10 Exclusive 10 OFF.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate molar mass in grams per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






