Your How to calculate liters per mole images are available in this site. How to calculate liters per mole are a topic that is being searched for and liked by netizens today. You can Download the How to calculate liters per mole files here. Download all free photos and vectors.
If you’re searching for how to calculate liters per mole pictures information related to the how to calculate liters per mole topic, you have visit the right site. Our site frequently gives you suggestions for refferencing the maximum quality video and image content, please kindly surf and locate more enlightening video articles and images that match your interests.
How To Calculate Liters Per Mole. Moles are defined as 60221023 individual units of a substance. Watch video tutorial When going from moles to liters you multiply by 224. 5 molar to mole per liter 5 mole per liter. 1 mol gas 22.
 Converting Between Moles And Liters Youtube From youtube.com
Converting Between Moles And Liters Youtube From youtube.com
Divide the number of moles of solute by the number of liters of solutionM0375 mol. Download Concentration - Molar Unit Converter our powerful software utility that helps you make easy conversion between more than 2100 various units of. A a M n V a a. N M V. You could also use moles per mL and other measures of volume. In chemistry and related fields the molar volume symbol V m or of a substance is the volume occupied by one mole of it at a given temperature and pressureIt is equal to the molar mass M divided by the mass density ρ.
Total number of moles 1 mole 585 g 6 g 062 moles Now determine moles per liter of solution.
Youll need to know the volume of water used. 10 molar to mole per liter 10 mole per liter. The key to the conversion is realizin. Divide the concentration mgml by the molecular weight. R 0082 057 338 47 L atm K1 mol1 that is a relative standard uncertainty of 57107 according to the 2014 CODATA recommended value 1. How to ger concentration molL of a solution from the mass grams of salt dissolved in water.
 Source: youtube.com
Source: youtube.com
50 molar to mole per liter 50 mole per liter. The relation is the molar volume. Bookmark molcubic centimeter to molliter Conversion Calculator - you will probably need it in the future. How To Calculate Moles Per Liter. Note that rounding errors may occur so always check the results.
 Source: youtube.com
Source: youtube.com
How do you convert moles to liters per molarity. This is enough to calculate the molarity. Youll need to know the volume of water used. Dimensional analysis of such problems can help you find what you need. Liters are a metric unit for volume.
 Source: youtube.com
Source: youtube.com
It has the SI unit of cubic metres per mole m 3 mol although it is typically more practical to use the units cubic decimetres per mole dm 3 mol for gases. Answered by Pearl Waters September 7 2021 As mass volume molarity molar mass then mass volume molar mass molarity. Molarity 020 M. Method 1 watch video tutorial. Without knowledge of the molarity molL or density kgL or gmL of the substance you cannot convert between the two.
 Source: abetterchemtext.com
Source: abetterchemtext.com
50 molar to mole per liter 50 mole per liter. At standard temperature and pressure STP one mole of an ideal. Answered by Pearl Waters September 7 2021 As mass volume molarity molar mass then mass volume molar mass molarity. Dimensional analysis of such problems can help you find what you need. The relation is the molar volume.
 Source: youtube.com
Source: youtube.com
1000 mmolL mM 1000000 µmolL µM 10 9 nmolL nM 10 12 pmolL pM. 20 molar to mole per liter 20 mole per liter. Molarity M or molL Number of Moles mol Volume L Example of molarity and concentration calculations The scenario in which most lab scientists will encounter this type of calculation is when making up solutions following a standard operating procedure SOP or a scientific paper. 1 molecubic meter is equal to 0001 mole per liters or 10E-6 moles per milliliters. Molarity 020 M.
 Source: youtube.com
Source: youtube.com
How many moles are in NaOH. Concentration is usually expressed in moles per liter which is called molarity. You could also use moles per mL and other measures of volume. 40 molar to mole per liter 40 mole per liter. Calculate the number of moles of solute presentmol NaOH150g NaOHx1 mol NaOH400 g NaOHmol NaOH0375 mol NaOH.
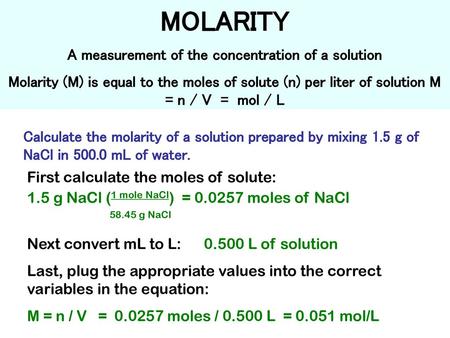 Source: slideplayer.com
Source: slideplayer.com
Divide the concentration mgml by the molecular weight. Use the conversion factor. 1000 mmolL mM 1000000 µmolL µM 10 9 nmolL nM 10 12 pmolL pM. Mole per liters or moles per milliliters. At standard temperature and pressure STP one mole of an ideal.
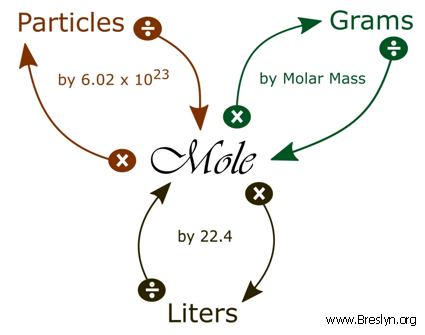 Source: breslyn.org
Source: breslyn.org
20 molar to mole per liter 20 mole per liter. Liters are a metric unit for volume. N M V. 1 mol gas 22. Molarity 020 M.
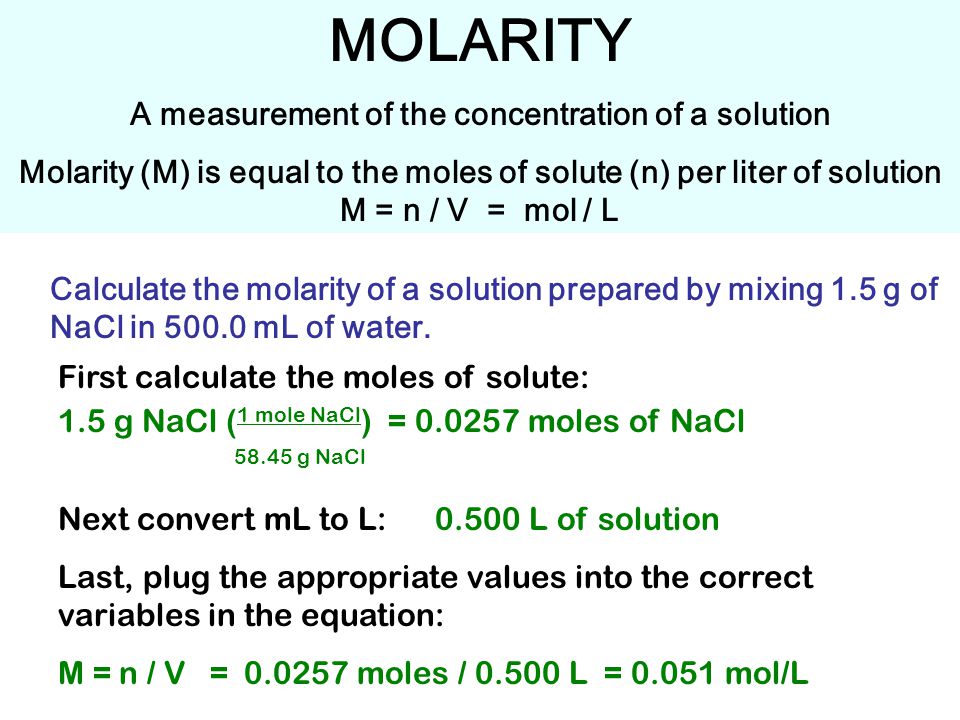 Source: slideplayer.com
Source: slideplayer.com
Note that rounding errors may occur so always check the results. Dimensional analysis of such problems can help you find what you need. Molarity 015 moles of KMnO 4 075 L of solution. Calculate the number of liters of solution presentL soln225 mLx1 L0225 L soln1000 mL. 1 mol gas 22.
 Source: slideplayer.com
Source: slideplayer.com
10 molar to mole per liter 10 mole per liter. Youll need to know the volume of water used. Moles are defined as 60221023 individual units of a substance. Moles mol x Molar Mass gmol 1 x 5844. How many moles are in NaOH.
 Source: slideplayer.com
Source: slideplayer.com
10 mgml 21 x 105 mgmmole. Molarity 020 M. In chemistry and related fields the molar volume symbol V m or of a substance is the volume occupied by one mole of it at a given temperature and pressureIt is equal to the molar mass M divided by the mass density ρ. Calculate the number of moles of solute presentmol NaOH150g NaOHx1 mol NaOH400 g NaOHmol NaOH0375 mol NaOH. This is enough to calculate the molarity.
 Source: nagwa.com
Source: nagwa.com
Mole per liters or moles per milliliters. Concentration is usually expressed in moles per liter which is called molarity. Download Concentration - Molar Unit Converter our powerful software utility that helps you make easy conversion between more than 2100 various units of. In chemistry and related fields the molar volume symbol V m or of a substance is the volume occupied by one mole of it at a given temperature and pressureIt is equal to the molar mass M divided by the mass density ρ. Dimensional analysis of such problems can help you find what you need.
 Source: khanacademy.org
Source: khanacademy.org
Calculate the number of liters of solution presentL soln225 mLx1 L0225 L soln1000 mL. Note that rounding errors may occur so always check the results. Liters are a metric unit for volume. Moles are defined as 60221023 individual units of a substance. A step-by-step explanation of how to convert from liters to molesIn this video were converting from liters to moles.
 Source: youtube.com
Source: youtube.com
M 062 moles NaCl 050 liter solution 12 M solution 12 molar solution. Liters are a metric unit for volume. 50 molar to mole per liter 50 mole per liter. Moles are defined as 60221023 individual units of a substance. At standard temperature and pressure STP one mole of an ideal.
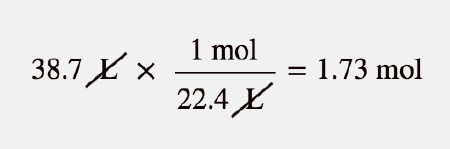 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Molarity moles of solute litres of solution. 20 molar to mole per liter 20 mole per liter. The Ideal Gas Law predicts very precisely not only gas volume but temp and the number of moles of gas. N M V. Mole per liters or moles per milliliters.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate liters per mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






