Your How to calculate grams to moles images are available in this site. How to calculate grams to moles are a topic that is being searched for and liked by netizens today. You can Get the How to calculate grams to moles files here. Download all free photos and vectors.
If you’re searching for how to calculate grams to moles pictures information connected with to the how to calculate grams to moles keyword, you have pay a visit to the right blog. Our site frequently gives you hints for seeking the maximum quality video and image content, please kindly surf and locate more enlightening video content and graphics that fit your interests.
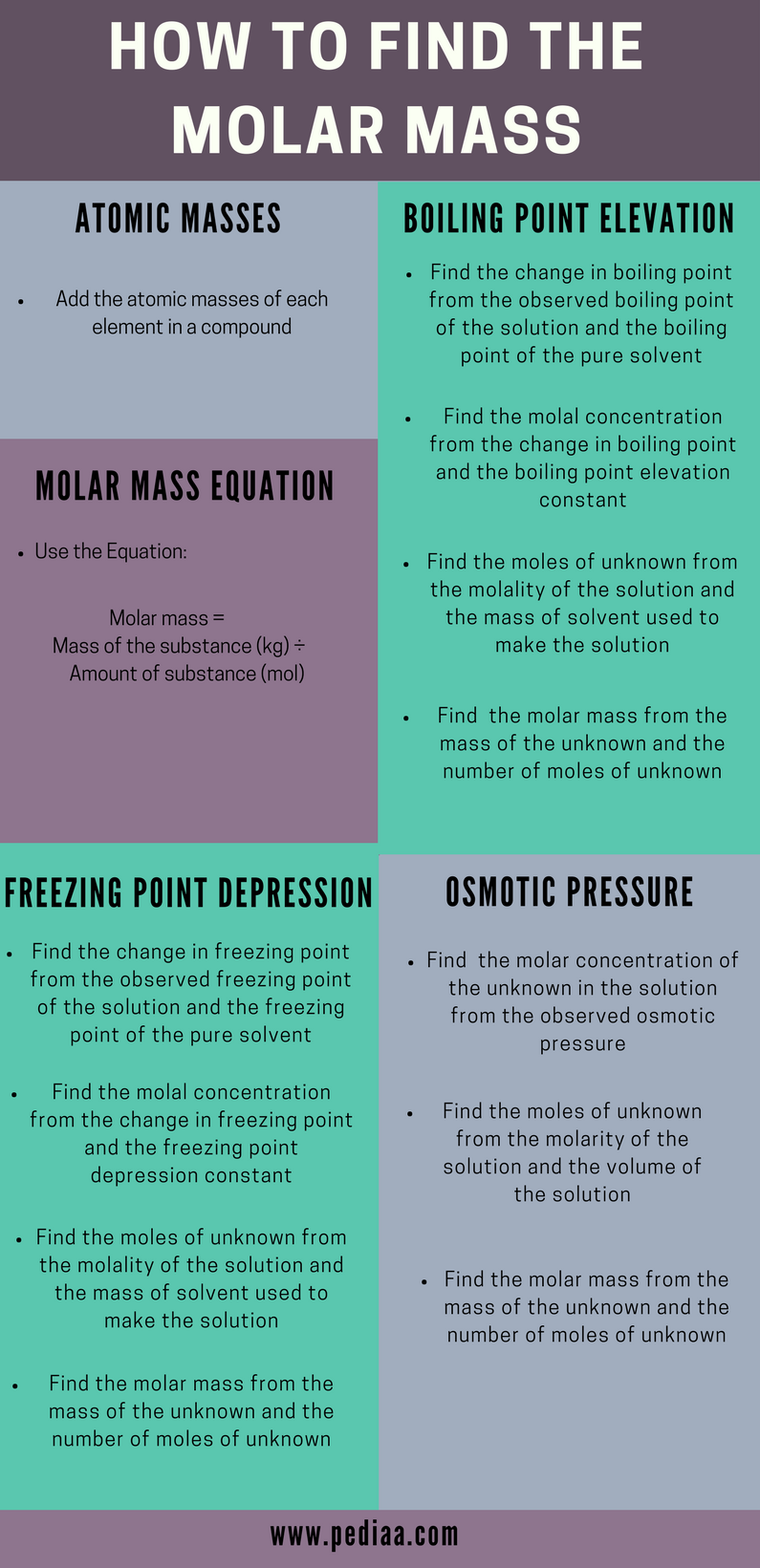
How To Calculate Grams To Moles. Compare the ratios to find the limiting reactant. Learn how to calculate theoretical yield easily. Take a look at the example of how to calculate moles from grams. Calculate molecular mass.
 Pin By Michelle M On Knowledge Molar Mass Teaching Chemistry Chemistry Lessons From pinterest.com
Pin By Michelle M On Knowledge Molar Mass Teaching Chemistry Chemistry Lessons From pinterest.com
The number of grams per mole for each single element is equal to the atomic weight of that element. To do this first calculate the molar mass of a sample. Therefore the number of. Learn how to calculate theoretical yield easily. Molecular weight of Sulfur or grams The molecular formula for Sulfur is S. Sometimes you then have to convert number of moles to grams.
Divide the moles of one reactant with the moles of the other to find the ratio of the 2 molecules then find the ideal ratio for the reaction.
How many moles Sulfur in 1 grams. 1 mole is equal to 1 moles Sulfur or. People can find the molar mass or atomic mass of elements on the periodic table and the molar mass of a compound is the total molar mass of all. 7 moles Silver to grams 7550774 grams. Molar mass of the substance. In 1 kg-moles of any substance there exists exactly 103.
 Source: pinterest.com
Source: pinterest.com
Molar mass details. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. In 1 kg-moles of any substance there exists exactly 103. One mole equals to a very large number of particles. Molar mass of the substance.
 Source: pinterest.com
Source: pinterest.com
Formula to convert moles to grams. Moles to Grams Example Problem. Divide the moles of one reactant with the moles of the other to find the ratio of the 2 molecules then find the ideal ratio for the reaction. Molar mass of the substance. Write down and identify the values.
 Source: pinterest.com
Source: pinterest.com
You can view more details on each measurement unit. People can find the molar mass or atomic mass of elements on the periodic table and the molar mass of a compound is the total molar mass of all. 1 mole is equal to 1 moles Sulfur or. 0700 mole x 340146 gramsmole 238 grams. 4 moles Silver to grams 4314728 grams.
 Source: pinterest.com
Source: pinterest.com
The SI base unit for amount of substance is the mole. Therefore the number of. Compare the ratios to find the limiting reactant. Take a look at the example of how to calculate moles from grams. 4 moles Silver to grams 4314728 grams.
 Source: pinterest.com
Source: pinterest.com
Find the molar mass of a water molecule H₂O. On the other hand if you are interested in mole calculation without using atoms to grams calculator follow the examples below. How many moles are in 16 grams of Oxygen gas. One mole equals to a very large number of particles. 2 moles Silver to grams 2157364 grams.
 Source: pinterest.com
Source: pinterest.com
Add up the masses of the elements in each compound to find the grams per mole for that compound. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams. One mole equals to a very large number of particles. The reason is that one mole of the substance contains the amount of moles that are exactly in 12 grams of the carbon-12. Then multiply it by the number of moles to get an answer in grams.
 Source: pinterest.com
Source: pinterest.com
This online calculator converts to moles given grams and converts to grams given moles All online calculators Articles Suggest a. The number of grams per mole for each single element is equal to the atomic weight of that element. Calculate the number of grams per mole gmol for each reactant and product. Divide the moles of one reactant with the moles of the other to find the ratio of the 2 molecules then find the ideal ratio for the reaction. To calculate or find the grams to moles or moles to grams the molar mass of each element will be used to calculate.
 Source: pinterest.com
Source: pinterest.com
2 The molar mass is 340146 gramsmole which is calculated using the formula and the atomic weights on a periodic table. How to calculate moles. 4 moles Silver to grams 4314728 grams. How many moles Sulfur in 1 grams. Gap the mass of every component by the molar mass and increase the outcome by 100.
 Source: pinterest.com
Source: pinterest.com
To do this first calculate the molar mass of a sample. Mass of the substance grams. People can find the molar mass or atomic mass of elements on the periodic table and the molar mass of a compound is the total molar mass of all. Learn how to calculate theoretical yield easily. Molar mass of the substance.
 Source: pinterest.com
Source: pinterest.com
Determine the moles in 200 grams of NaCl. The number of grams per mole for each single element is equal to the atomic weight of that element. Moles to Grams Example Problem. Multiply the moles given by the substances molar mass. On the other hand sometimes youre given a value in moles and need to convert it to grams.
 Source: pinterest.com
Source: pinterest.com
2 moles Silver to grams 2157364 grams. Mass of the substance grams. 5 moles Silver to grams 539341 grams. How many moles are there in 1kg. 1 170 grams are given in the text of the problem.
 Source: pinterest.com
Source: pinterest.com
You can find the. Molecular weight of Sulfur or grams The molecular formula for Sulfur is S. On the other hand if you are interested in mole calculation without using atoms to grams calculator follow the examples below. Take a look at the example of how to calculate moles from grams. To convert moles to g we multiply the moles by the molar mass also known as the molecular weight.
 Source: pinterest.com
Source: pinterest.com
People can find the molar mass or atomic mass of elements on the periodic table and the molar mass of a compound is the total molar mass of all. Then multiply it by the number of moles to get an answer in grams. Calculate the number of grams per mole gmol for each reactant and product. Divide the moles of one reactant with the moles of the other to find the ratio of the 2 molecules then find the ideal ratio for the reaction. Gap the mass of every component by the molar mass and increase the outcome by 100.
 Source: pinterest.com
Source: pinterest.com
0700 mole x 340146 gramsmole 238 grams. Therefore the number of. 5 moles Silver to grams 539341 grams. Converting grams to moles is super easy if you use mass to moles calculator above. Since we know the molar mass of NaCl is 5844gmol therefore we will multiply it by 02 to get grams.
 Source: pinterest.com
Source: pinterest.com
Mass of the substance grams. Divide the moles of one reactant with the moles of the other to find the ratio of the 2 molecules then find the ideal ratio for the reaction. The number of grams per mole for each single element is equal to the atomic weight of that element. The ideal gas law is PV nRT so if you know enough values you can calculate volume V or the number of moles n. Take a look at the example of how to calculate moles from grams.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate grams to moles by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.