Your How to calculate gram molecular weight of naoh images are available. How to calculate gram molecular weight of naoh are a topic that is being searched for and liked by netizens today. You can Find and Download the How to calculate gram molecular weight of naoh files here. Download all royalty-free images.
If you’re searching for how to calculate gram molecular weight of naoh images information connected with to the how to calculate gram molecular weight of naoh keyword, you have pay a visit to the right site. Our site frequently provides you with hints for viewing the maximum quality video and picture content, please kindly hunt and find more informative video content and images that match your interests.
How To Calculate Gram Molecular Weight Of Naoh. Calculate the mass of sodium hydroxide required to make 500ml of 010 M solution. The molar mass of NaOH 40g. It is the amount of methane that contains 4 602 1023 hydrogen atoms. Molarity refers to the number of moles of the solute present in 1 liter of solution.
 Pin By Maria Mihoc On Science 7 Element Symbols Year 7 Science Science From pinterest.com
Pin By Maria Mihoc On Science 7 Element Symbols Year 7 Science Science From pinterest.com
The concepts of equivalent weight and gram equivalents help link the concept of molar ratios to the fact that different elements have different molecular weights. This quantity of molecules is referred to as a mole. A molar solution has its concentration expressed as the number of gram-molecular masses moles of substance per liter. The molar mass of NaOH 40g. The density of 85 ww Phosphoric acid is 1685 gml at 25C which means that the weight of the 1 ml of Phosphoric acid is 1685 gram at 25C. If you know that how many grams of NaOH is dissolved in known volume of solvent then u can determine its molarity by using below formula.
Some acids can donate more than one proton introducing a difference between molecular weight and equivalent weight for these compounds.
If you know that how many grams of NaOH is dissolved in known volume of solvent then u can determine its molarity by using below formula. Because NaOH has one OH ion that reacts the equivalent weight is 40 geq. Calculate the mass of sodium hydroxide required to make 500ml of 010 M solution. BYJUS provides accurate solutions prepared by our specialised experts. Calculate the mass of FeSO 4. It is the amount of methane that contains 4 602 1023 hydrogen atoms.
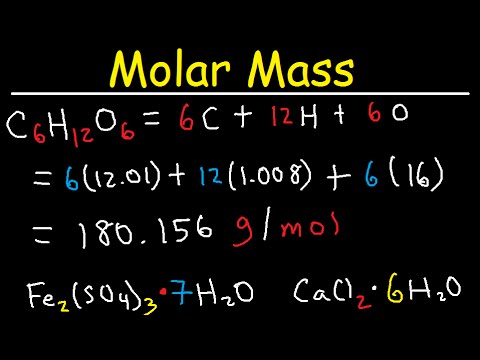 Source: youtube.com
Source: youtube.com
Volume of NaOH 500ml 05 L. Respirable quartz weight of quartz mg or μg x 100 sample respirable dust weight mg or μg Calculate the PEL for the sample using the reported percent respirable quartz entered as a whole number eg if the quartz is 7 use the whole number 7 in Equation 6. Normality is defined as the gram equivalent weight per liter of solution. The SI base unit for amount of substance is the mole. Calculate the mass of FeSO 4.
 Source: toppr.com
Source: toppr.com
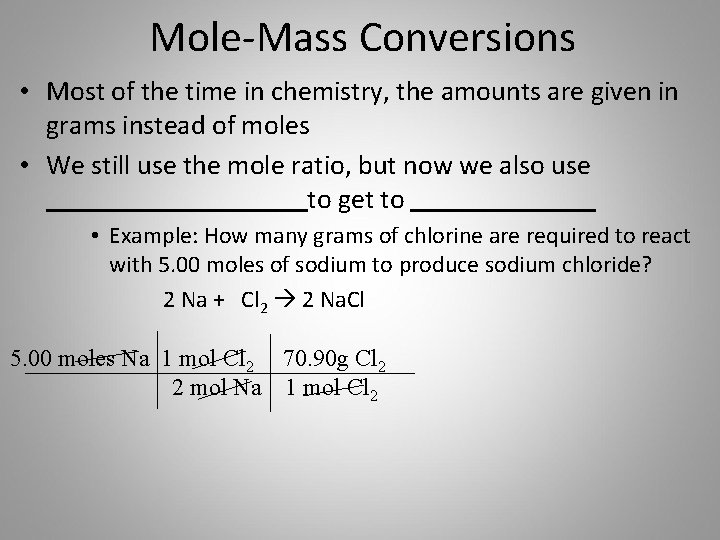
Molarity moles volume in litres weight of NaOH molarity x. 10 gm of NaOH is dissolved in 250 ml of water then the molarity of NaOH is calculated by Molarity Weight of NaOH taken 10. Normality is defined as the gram equivalent weight per liter of solution. Answer 1 of 6. Molarity refers to the number of moles of the solute present in 1 liter of solution.
 Source: pinterest.com
Source: pinterest.com
For example to prepare a 1N NaCl solution you need to dissolve 40g of NaCl in 1000mL of water. Calculate the mass of sodium hydroxide required to make 500ml of 010 M solution. 1 mole is equal to 1 moles NaOH or 3999711 grams. Normality N expresses a concentration of a solution. In order to solve a normality calculation for NaOH the equivalent weight must be calculated.
 Source: wikihow.com
Source: wikihow.com
Molecular weight of H 2 SO 4 98. Answer 1 of 6. The molar mass of NaOH 40g. Molecular weight of NaOH or grams This compound is also known as Sodium Hydroxide. The concepts of equivalent weight and gram equivalents help link the concept of molar ratios to the fact that different elements have different molecular weights.
 Source: pinterest.com
Source: pinterest.com
Mass Relations in Chemical Reactions Homework ____ 7. 10 gm of NaOH is dissolved in 250 ml of water then the molarity of NaOH is calculated by Molarity Weight of NaOH taken 10. You can also use the formula Normality weight of substance in Grams 1000 equivalent weightvolume required in mL. For example to prepare a 1N NaCl solution you need to dissolve 40g of NaCl in 1000mL of water. Molecular weight of H 2 SO 4 98.
 Source: youtube.com
Source: youtube.com
The SI base unit for amount of substance is the mole. For example to prepare a 1N NaCl solution you need to dissolve 40g of NaCl in 1000mL of water. Mass Relations in Chemical Reactions Homework ____ 7. BYJUS provides accurate solutions prepared by our specialised experts. Normality N expresses a concentration of a solution.
 Source: pinterest.com
Source: pinterest.com
Normality N expresses a concentration of a solution. You can view more details on each measurement unit. Some acids can donate more than one proton introducing a difference between molecular weight and equivalent weight for these compounds. BYJUS provides accurate solutions prepared by our specialised experts. A molar solution has its concentration expressed as the number of gram-molecular masses moles of substance per liter.
 Source: youtube.com
Source: youtube.com
Molecular weight of H 2 SO 4 98. The molar mass of NaOH 40g. Calculate the mass of FeSO 4. 1 mole is equal to 1 moles NaOH or 3999711 grams. Answer 1 of 6.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles NaOH or 3999711 grams. Molecular weight of NaOH or grams This compound is also known as Sodium Hydroxide. Mass Relations in Chemical Reactions Homework ____ 7. Molecular weight of H 2 SO 4 98. Respirable quartz weight of quartz mg or μg x 100 sample respirable dust weight mg or μg Calculate the PEL for the sample using the reported percent respirable quartz entered as a whole number eg if the quartz is 7 use the whole number 7 in Equation 6.
 Source: wikihow.com
Source: wikihow.com
BYJUS provides accurate solutions prepared by our specialised experts. The molar mass of NaOH 40g. 10 gm of NaOH is dissolved in 250 ml of water then the molarity of NaOH is calculated by Molarity Weight of NaOH taken 10. If you know that how many grams of NaOH is dissolved in known volume of solvent then u can determine its molarity by using below formula. Molecular weight of H 2 SO 4 98.
 Source: wikihow.com
Source: wikihow.com
Molecular weight of H 2 SO 4 98. The chapter contains important topics such as atomic and molecular mass molar mass gram atomic mass and gram molecular mass. You can view more details on each measurement unit. Mass Relations in Chemical Reactions Homework ____ 7. A molar solution has its concentration expressed as the number of gram-molecular masses moles of substance per liter.
 Source: wikihow.com
Source: wikihow.com
Mass Relations in Chemical Reactions Homework ____ 7. Calculate the mass of FeSO 4. Which of the following is not a correct description of 160 grams of methane CH 4. 1 mole is equal to 1 moles NaOH or 3999711 grams. The chapter contains important topics such as atomic and molecular mass molar mass gram atomic mass and gram molecular mass.
 Source: wikihow.com
Source: wikihow.com
BYJUS provides accurate solutions prepared by our specialised experts. The molecular mass of any compound in grams gram-molecular mass contains the same number of molecules Avogadros number 602x1023. Because NaOH has one OH ion that reacts the equivalent weight is 40 geq. The density of 85 ww Phosphoric acid is 1685 gml at 25C which means that the weight of the 1 ml of Phosphoric acid is 1685 gram at 25C. BYJUS provides accurate solutions prepared by our specialised experts.
 Source: youtube.com
Source: youtube.com
Normality N expresses a concentration of a solution. Of moles volume mass of solute98 95100183498. This quantity of molecules is referred to as a mole. Because NaOH has one OH ion that reacts the equivalent weight is 40 geq. For example to prepare a 1N NaCl solution you need to dissolve 40g of NaCl in 1000mL of water.
 Source: pinterest.com
Source: pinterest.com
Which of the following is not a correct description of 160 grams of methane CH 4. The molecular mass of any compound in grams gram-molecular mass contains the same number of molecules Avogadros number 602x1023. The molar mass of NaOH 40g. You can also use the formula Normality weight of substance in Grams 1000 equivalent weightvolume required in mL. Of moles volume mass of solute98 95100183498.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate gram molecular weight of naoh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.