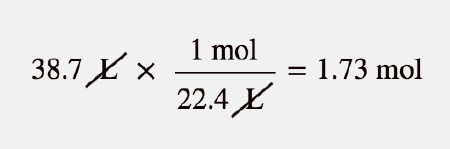Your How to calculate gram molecular mass of methane images are ready in this website. How to calculate gram molecular mass of methane are a topic that is being searched for and liked by netizens now. You can Get the How to calculate gram molecular mass of methane files here. Find and Download all free images.
If you’re searching for how to calculate gram molecular mass of methane images information related to the how to calculate gram molecular mass of methane interest, you have pay a visit to the ideal site. Our site frequently provides you with suggestions for downloading the maximum quality video and picture content, please kindly hunt and find more enlightening video articles and images that match your interests.
How To Calculate Gram Molecular Mass Of Methane. 1 grams Methane 0062334579609362 mole using the molecular weight calculator and the molar mass of CH4. Molecular mass of methane CH 4 12 x 1 1 x 4 12 4 16 g. The molar mass of KMnO4 is 158032 gmol. The molecular weight of CH4 is c 4h.
 Calculate Molecular Mass Of The Following Molecules A Sulphuric Acid H 2 So 4 B Gluco Youtube From youtube.com
Calculate Molecular Mass Of The Following Molecules A Sulphuric Acid H 2 So 4 B Gluco Youtube From youtube.com
One mole of methane equals 1604 grams because a molecule of methane has an atomic weight of 1604. Since 1 mole is 6022 x 1023 you are given 5 moles of CH4. 120107 1007944 Percent composition by element. Grams Moles and Molecular Mass Evaluate 1. So the overall solution for this problem is to use molar mass of CH_4 methane to convert grams of methane into moles of methane. Number of molecules 0499 mol 6022 1023 1mol 3005 1023 molecules.
You can view more details on each measurement unit.
The molecular weight of CH4 is c 4h. N 80 gr 16043 grmol 0499 mol. Its submitted by government in the best field. The equation shows that 16 g of methane requires 64 g of Oxygen. There are 4 hydrogen atoms in methane. One mole of methane equals 1604 grams because a molecule of methane has an atomic weight of 1604.
 Source: studylib.net
Source: studylib.net
Molecular mass of oxygen 16. Hence we can conclude that n number of moles in 16 g of methane is 1 mol. Mass of 2 molecules of oxygen 64 g. There are total 4 bonds between C and H atom. CaCO3 is the only excess reactant in this example so the amount.
 Source: youtube.com
Source: youtube.com
24 moles of sulphur dioxide. The molar mass of the methane is M1604gmol M 1604 g m o l. Density of methane gas is equal to 0554 kgm³. 24 moles of sulphur dioxide. Molecular weight of Methane or mol The molecular formula for Methane is CH4.
 Source: slideplayer.com
Source: slideplayer.com
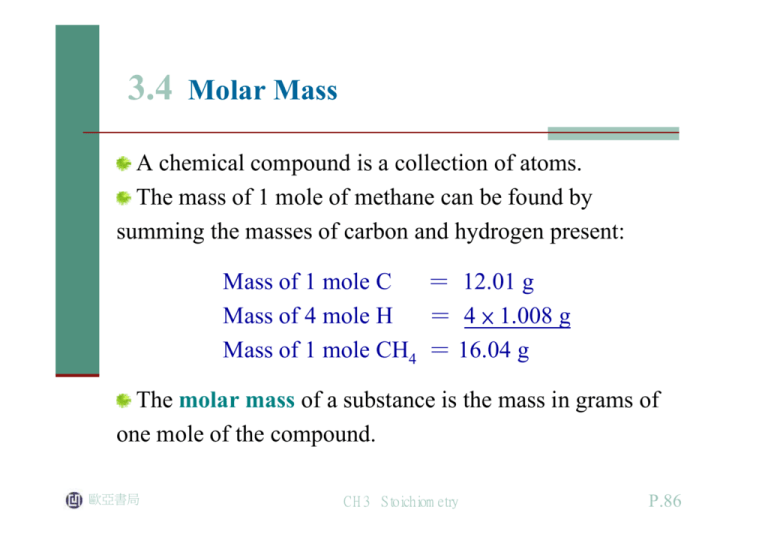
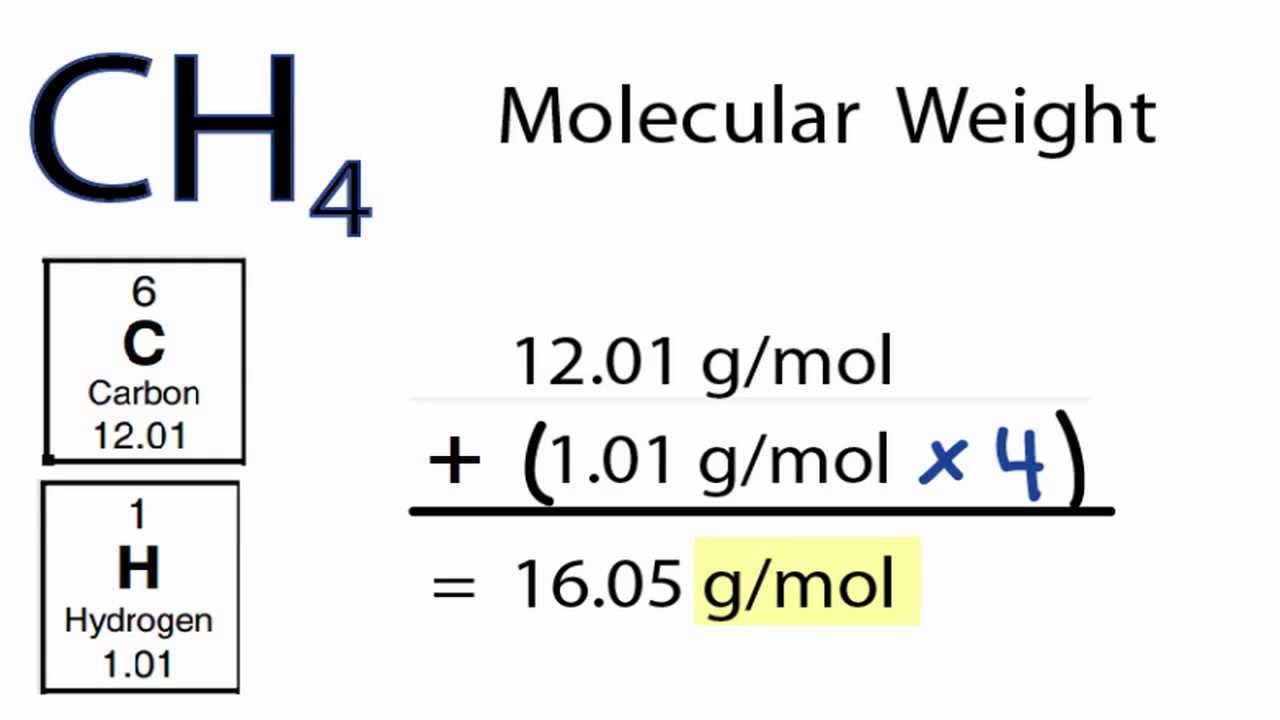
In other words carbon constitutes 7487 of the mass of methane. So 376 10 23 hydrogen atoms are there in 250 g of methane. Hence we can conclude that n number of moles in 16 g of methane is 1 mol. The atomic weight of carbon is 1201 grams per mole. Molar mass of CH4 1604246 gmol.
 Source: youtube.com
Source: youtube.com
Since 1 mole is 6022 x 1023 you are given 5 moles of CH4. By the same token 404 of the 3604 grams of water formed in reaction 1 was hydrogen. Molecular weight of Methane or mol The molecular formula for Methane is CH4. The molar mass of the methane is M1604gmol M 1604 g m o l. Number of moles of sulphur dioxide 24 n.
 Source: youtube.com
Source: youtube.com
At 0C 32F or 27315K at commonplace atmospheric stress. Molecular weight of CH4 M CH4 16043. Weight is equal to molecular weight multiplied by the number of moles. 34 g x 1mol160424g 021 moles C. 1 CH4 Molecule equals 1 C atom and 4 H atoms.
 Source: toppr.com
Source: toppr.com
From the formula of methane it is clear that one mole of methane has 4 hydrogen atoms. By the same token 404 of the 3604 grams of water formed in reaction 1 was hydrogen. Number of moles of methane 35 n. Since all of the hydrogen in the water was originally in part of the methane we can calculate that hydrogen constituted 2513 of the mass of the methane. CaCO3 is the only excess reactant in this example so the amount.
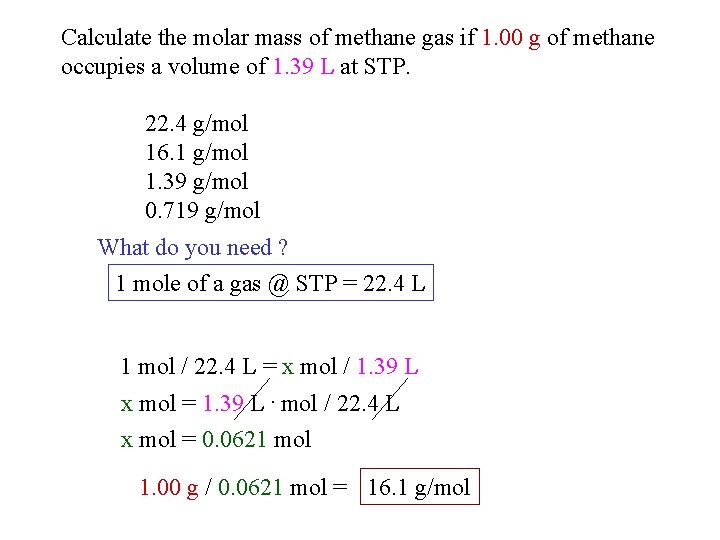 Source: slidetodoc.com
Source: slidetodoc.com
Mass of methane 35 16 56 g. 1 grams Methane 0062334579609362 mole using the molecular weight calculator and the molar mass of CH4. At 0C 32F or 27315K at commonplace atmospheric stress. Molar Mass of methane CH4. 1 grams Methane is equal to 0062334579609362 mole.
 Source: wikihow.com
Source: wikihow.com
After you have found the number of moles of methane CH4 you should multiply this number by avogadro number 6022 1023 and you get the number of molecules. So the overall solution for this problem is to use molar mass of CH_4 methane to convert grams of methane into moles of methane. From the formula of methane it is clear that one mole of methane has 4 hydrogen atoms. Grams Moles and Molecular Mass Evaluate 1. The atomic weight of carbon is 1201 grams per mole.
 Source: youtube.com
Source: youtube.com
From the formula of methane it is clear that one mole of methane has 4 hydrogen atoms. Since 1 mole is 6022 x 1023 you are given 5 moles of CH4. Methane gas weighs 0000554 gram per cubic centimeter or 0554 kilogram per cubic meter ie. At 0C 32F or 27315K at standard atmospheric pressure. Molar Mass of methane CH4.
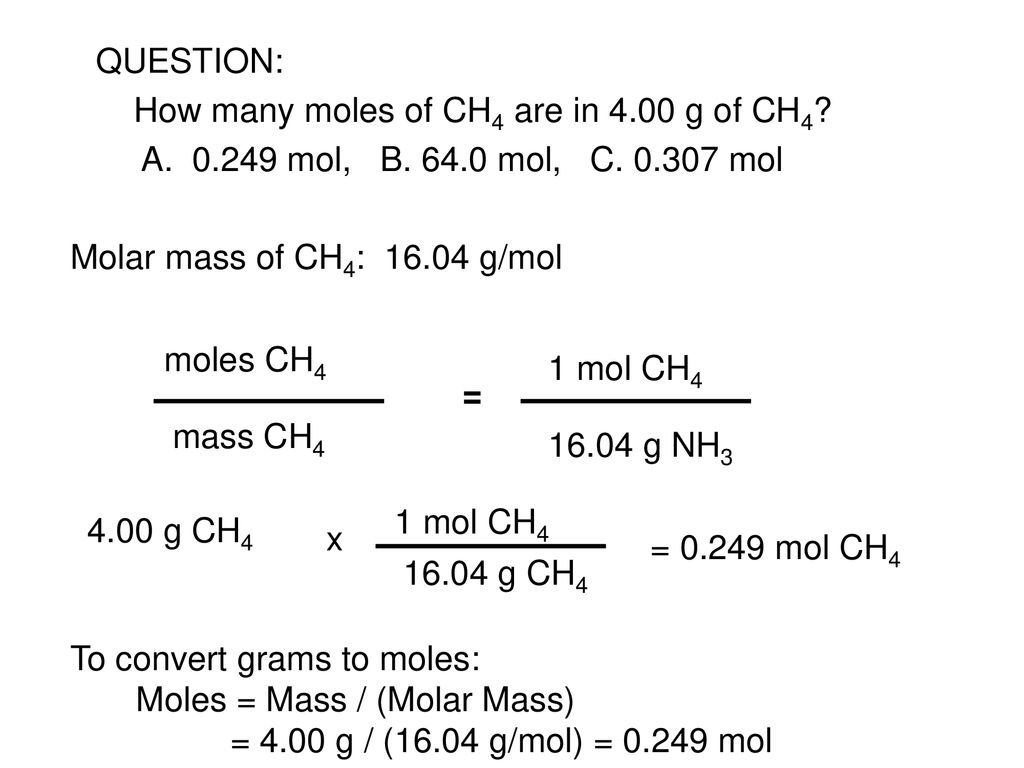 Source: slideplayer.com
Source: slideplayer.com
We undertake this kind of Calculate Molecular Weight graphic could possibly be the most trending subject once we share it in google gain or facebook. 24 moles of sulphur dioxide. To calculate the molar mass add the mass of each element The molecular formula of methane is CH4. N 80 gr 16043 grmol 0499 mol. 1 CH4 Molecule equals 1 C atom and 4 H atoms.
 Source: youtube.com
Source: youtube.com
Mass of Methane 16 g. Molecular weight of CH4 M CH4 16043. N 80 gr 16043 grmol 0499 mol. Since 1 mole is 6022 x 1023 you are given 5 moles of CH4. Methane molecular weight.
 Source: youtube.com
Source: youtube.com
To calculate the molar mass add the mass of each element The molecular formula of methane is CH4. Molecular mass ofCO 2 Molecular mass of carbon 2 Molecular mass of oxygen 2 atoms of oxygen are present Molecular mass of carbon 12. Here mole number of CH4 m CH4 093. Mass of 2 molecules of oxygen 64 g. So the overall solution for this problem is to use molar mass of CH_4 methane to convert grams of methane into moles of methane.
 Source: wikihow.com
Source: wikihow.com
Number of moles of sulphur dioxide 24 n. So its molecular weight is 12 4 16. Molecular mass ofCO 2 Molecular mass of carbon 2 Molecular mass of oxygen 2 atoms of oxygen are present Molecular mass of carbon 12. 1 cubic centimeter of methane gas weighs 0000554 gram g 1 cubic inch of methane gas weighs 0000320232 ounce oz Methane gas weighs 0000554 gram per cubic centimeter or 0554 kilogram per cubic meter ie. Density of methane gas is equal to 0554 kgm³.

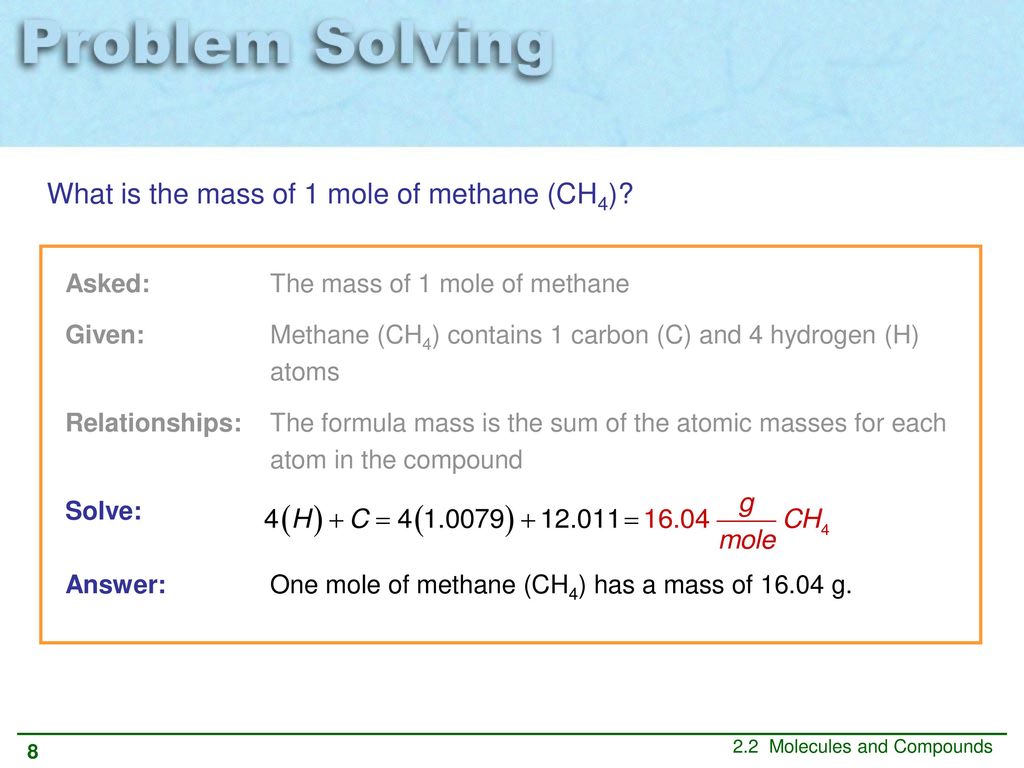
The SI base unit for amount of substance is the mole. So the overall solution for this problem is to use molar mass of CH_4 methane to convert grams of methane into moles of methane. You can view more details on each measurement unit. 1008 4 4032 u displaystyle 100844032u. 1 Carbon 1 X 1201 gmole 1201 gmole 4 Hydrogens 4 X 101 gmole 1605 gmole which is the molar mass.
 Source: toppr.com
Source: toppr.com
Its submitted by government in the best field. By the same token 404 of the 3604 grams of water formed in reaction 1 was hydrogen. 1 CH4 Molecule equals 1 C atom and 4 H atoms. Its submitted by government in the best field. The molecular weight of CH4 is c 4h.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate gram molecular mass of methane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.