Your How to calculate concentration of h2o2 images are available in this site. How to calculate concentration of h2o2 are a topic that is being searched for and liked by netizens today. You can Get the How to calculate concentration of h2o2 files here. Download all royalty-free photos and vectors.
If you’re searching for how to calculate concentration of h2o2 images information connected with to the how to calculate concentration of h2o2 interest, you have visit the right site. Our website frequently gives you hints for viewing the highest quality video and image content, please kindly surf and locate more enlightening video articles and graphics that fit your interests.
How To Calculate Concentration Of H2o2. Therefore the volume strngth of 1 L of original H2O2 solution will be 142 L. Use a pipet and transfer 10 mL of solution 3 to beaker 1. Calculate the number of moles of MnO. 100 x 400 x 03125 so 400 x 03125100 125 M.
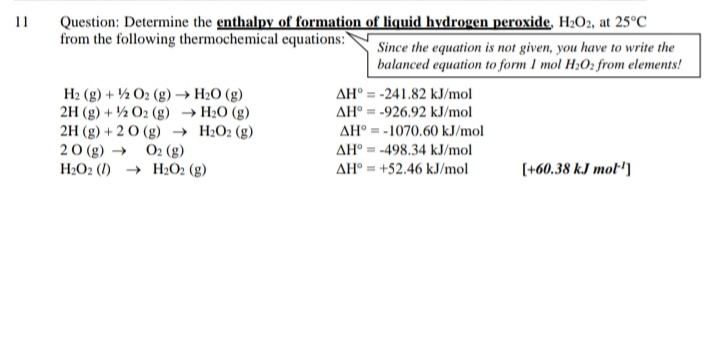 Answered Question Determine The Enthalpy Of Bartleby From bartleby.com
Answered Question Determine The Enthalpy Of Bartleby From bartleby.com
Dissolve about 6 ml of 30 hydrogen peroxide in 500 ml distilled water. As O2 is a gas it makes more sense to work with the number of moles than with concentration to obtain the concentration of H2O2. 35 Desired Dilution To. Amount of 35 H2O2 to add to container in ml. Acetic acid content material VEQ. Use a pipet and transfer 10 mL of solution 3 to beaker 1.
But if you only have a basic concentration dilution calculator in hand it is possible to measure it as well.
Hydrogen peroxide dilution calculator is a simple online tool that you can use to calculate the ratio of this substance to water. Direct measurement of the absorbance at 240nm of the H2O2 molecule. 2 eg about 20 g for 1 H. Through reaction of the peroxide with ferrous iron monitored via a subsequent reaction with the dye xylenol orange and measurement of the absorbance of the solution at 550nm. M1 concentration of H2O2 before mixing contents V1 volume of H2O2. For example ½ cup of water and ¼ cup of 3 hydrogen peroxide ¾ cup of 1 hydrogen peroxide.

Calculate the initial concentration for each solution and the reaction rate. Weigh a 50-mL beaker to the nearest 01 mg. 33 Calculating the percentage concentration weightvolume of H2O2 solution Percent Concentration Weight volume 100 X Amount of solute in grams. Stop the timer when the solutions color changes. Portfolio there is also a hydrogen peroxide solution sold as a disinfection.
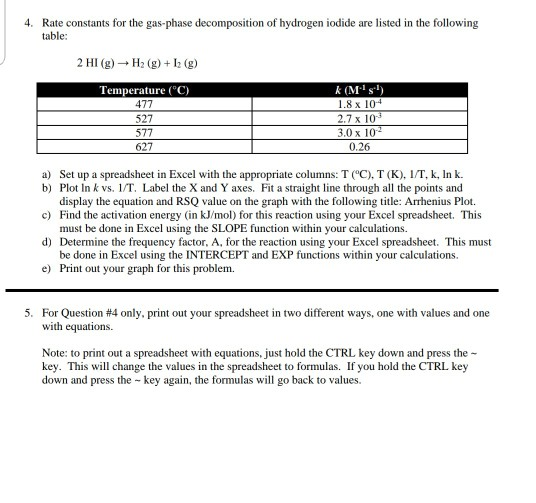
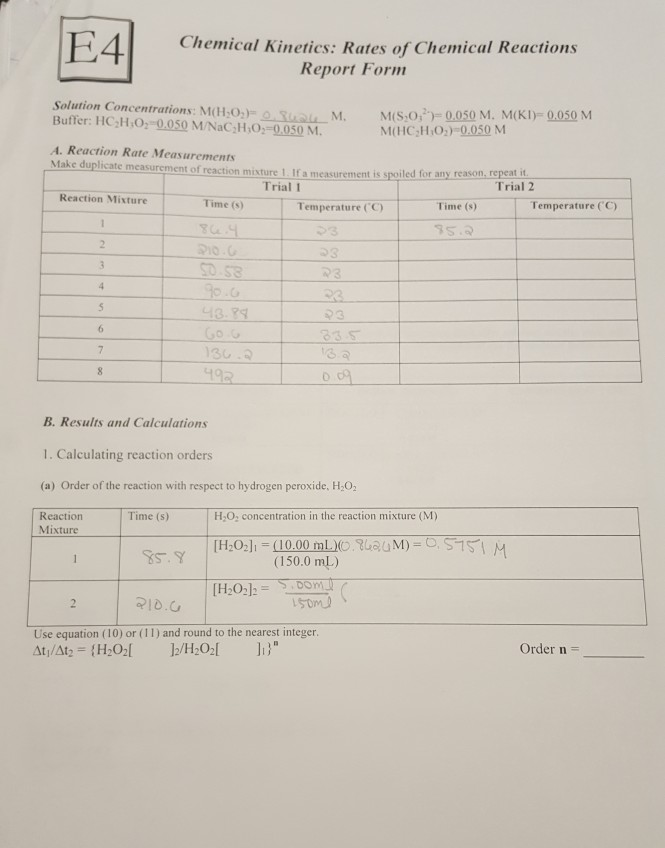 Source: chegg.com
Source: chegg.com
N the order of H2O2 in the first reaction. Solution 3 H2O2 is found in beaker 3. Dissolve about 6 ml of 30 hydrogen peroxide in 500 ml distilled water. 10 Volume of H 2 O 2 means that 1L at H 2 O 2 at STP gives 10L of oxygen at STP2H 2 O 2 l O 2 gH 2 Ol234 g 224 L at STP68 g227L of oxygen produced from 68gmH 2 O 2 10L of O 2 at STP is produced from 2246810 g3036 g30 gH 2 OThe strength of 10 Volume H 2 O 2 30 gL3 H 2 O 2 solutionHence the correct option is A. 33 Calculating the percentage concentration weightvolume of H2O2 solution Percent Concentration Weight volume 100 X Amount of solute in grams.
 Source: chegg.com
Source: chegg.com
The classical methods for measuring hydrogen peroxide concentration. In this experiment we determine n and m as well as k1. Quantity of water so as to add to container in ml. Enter the solution strength of your second solution. But if you only have a basic concentration dilution calculator in hand it is possible to measure it as well.
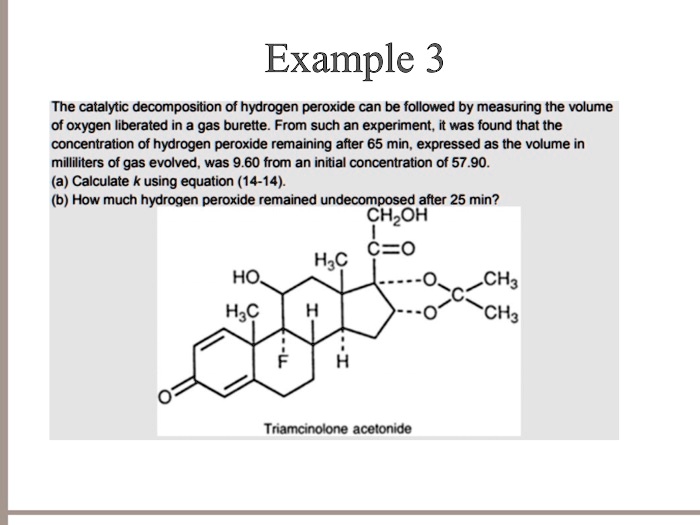 Source: numerade.com
Source: numerade.com
The introduction states that the hydrogen peroxide solution sold in grocery stores is 3 by mass. Hence the concentration of original solution is. 33 Calculating the percentage concentration weightvolume of H2O2 solution Percent Concentration Weight volume 100 X Amount of solute in grams. M1 concentration of H2O2 before mixing contents V1 volume of H2O2. Calculate the number of moles of MnO.
 Source: youtube.com
Source: youtube.com
Weigh a 50-mL beaker to the nearest 01 mg. Enter the strength of this solution often 35. X h mole fraction of H 2 O 2. However you probably dont want to weigh out. Actively helping customers employees and the global community during the coronavirus SARS-CoV-2 outbreak.
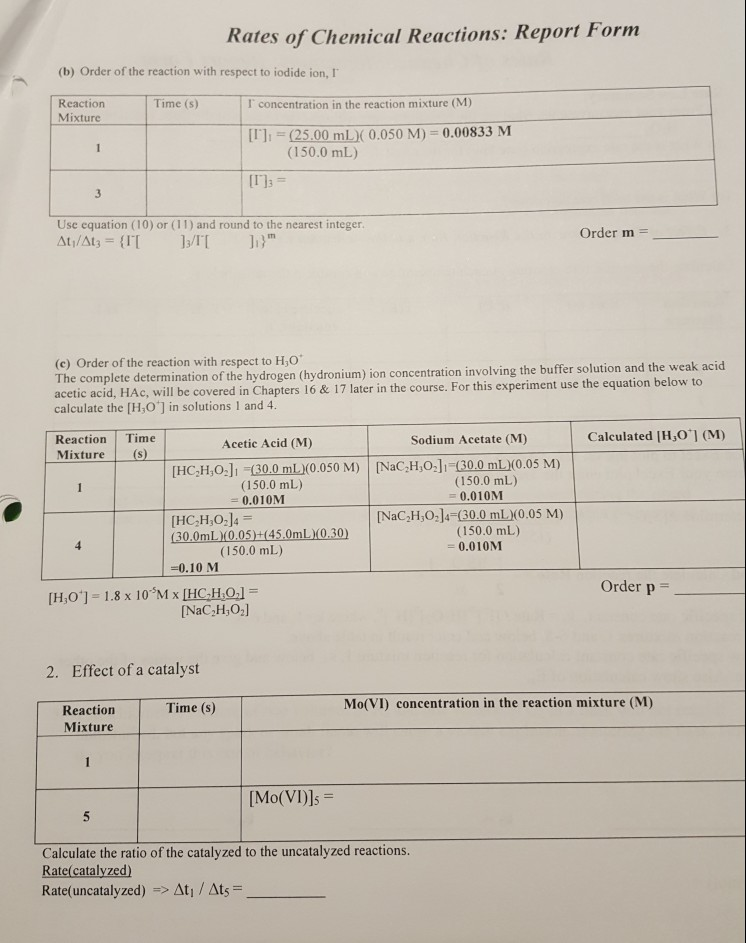
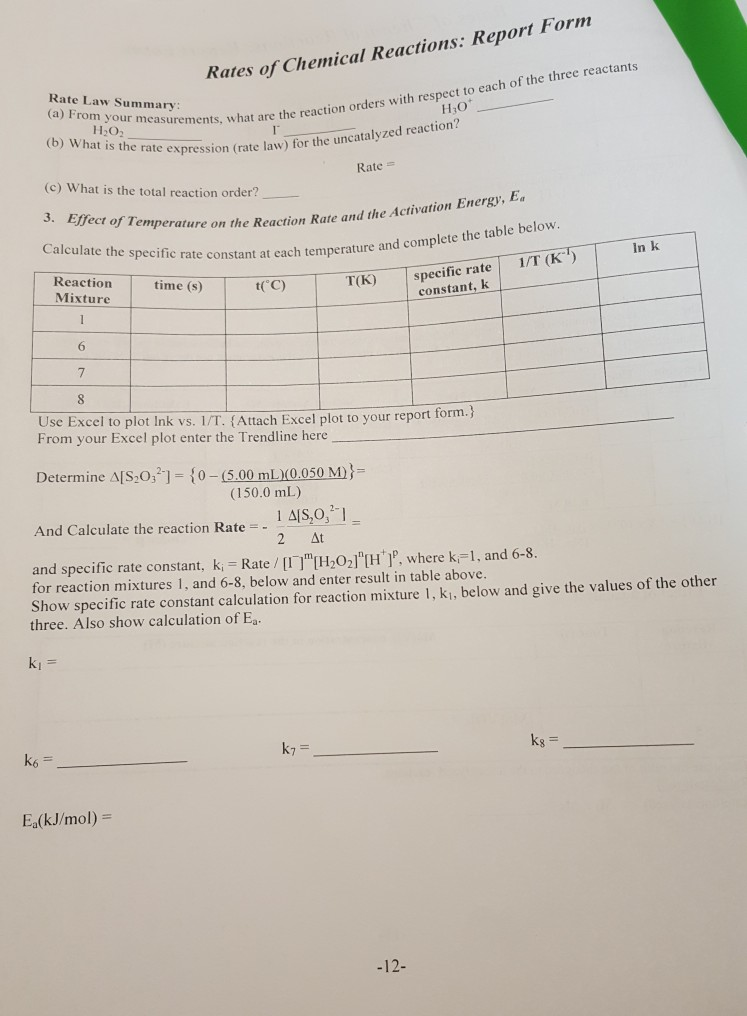 Source: chegg.com
Source: chegg.com
2 a proportionally larger sample for residual H. Hence the concentration of original solution is. Through reaction of the peroxide with ferrous iron monitored via a subsequent reaction with the dye xylenol orange and measurement of the absorbance of the solution at 550nm. In 201 mL of 0020. 125 moles 125 x 34 425 g of H2O2.
 Source: chegg.com
Source: chegg.com
35 Desired Dilution To. Calculate the number of moles of hydrogen peroxide needed to react with 205 mL of 0020. The classical methods for measuring hydrogen peroxide concentration are. Enter the strength of this solution often 35. 35 Desired Dilution To.
 Source: textilelearner.net
Source: textilelearner.net
M w molecular weight of water 18016 M h molecular weight of H 2 O 2 34016 W weight of H 2 O 2. Calculate the number of moles of hydrogen peroxide needed to react with 205 mL of 0020. Calculate the number of moles of MnO. You might use the precise focus of the lot to calculate molarity extra exactly. Therefore the volume strngth of 1 L of original H2O2 solution will be 142 L.
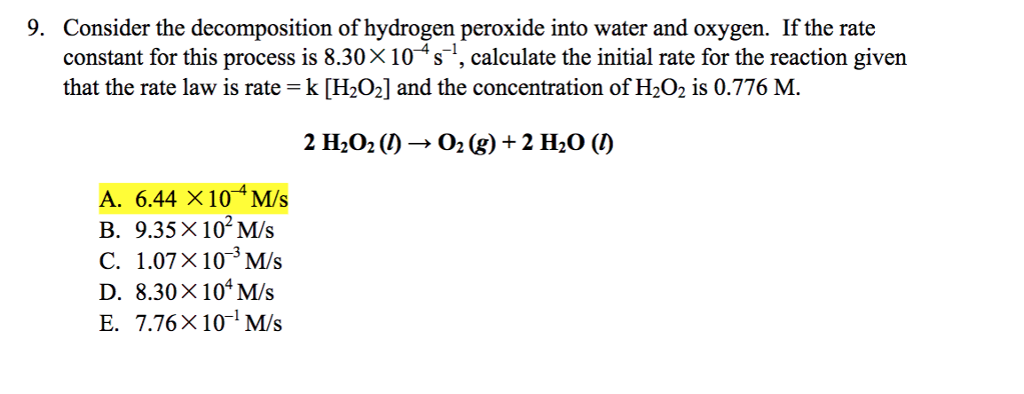 Source: chegg.com
Source: chegg.com
Sold solution should be of 3 ww. Popular Answers 1 21st Sep 2017. You might use the precise focus of the lot to calculate molarity extra exactly. Quantity of water so as to add to container in ml. So we obtain 00176328057728 milimoles of permanganate.
 Source: chegg.com
Source: chegg.com
However you probably dont want to weigh out. Then 425 g H2O2 will give 142 L of Oxygen gas. How to the Use Calculator. Direct measurement of the absorbance at 240nm of the H2O2 molecule. If your solution is 30 ww and you want 3 take for example 1 kg H2O2 add 9 kg H2O making 10 kg total of 3 ww.
 Source: toppr.com
Source: toppr.com
Similar questions and discussions. 2 eg about 20 g for 1 H. 2 moles 2 x 34 68 g of H2O2 gives 227 L of Oxygen gas. I like to express the milimoles by directly multiplying the molarity and titer. How to the Use Calculator.
 Source: bartleby.com
Source: bartleby.com
However you probably dont want to weigh out. Therefore the volume strngth of 1 L of original H2O2 solution will be 142 L. Enter the solution strength of your second solution. Calculate the initial concentration for each solution and the reaction rate. Enter the desired final dilution.
 Source: researchgate.net
Source: researchgate.net
K1 the rate constant of the first reaction. 3 Total Diluted Volume. Through reaction of the peroxide with ferrous iron monitored via a subsequent reaction with the dye xylenol orange and measurement of the absorbance of the solution at 550nm. Determination of Hydrogen Peroxide. 2 a proportionally larger sample for residual H.
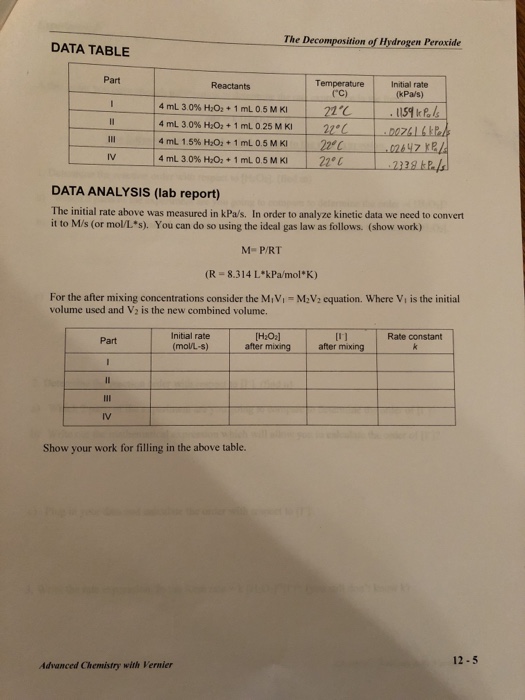
2 moles 2 x 34 68 g of H2O2 gives 227 L of Oxygen gas. Feel free to use our model to calculate from concentration by weight to the corresponding density of hydrogen peroxide. As O2 is a gas it makes more sense to work with the number of moles than with concentration to obtain the concentration of H2O2. Then 425 g H2O2 will give 142 L of Oxygen gas. In this experiment we determine n and m as well as k1.
 Source: chegg.com
Source: chegg.com
M the order of I in the first reaction. Enter the solution strength of your second solution. Dissolve about 6 ml of 30 hydrogen peroxide in 500 ml distilled water. Portfolio there is also a hydrogen peroxide solution sold as a disinfection. Acetic acid content material VEQ.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate concentration of h2o2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






