Your How to calculate concentration in moles per litre images are available in this site. How to calculate concentration in moles per litre are a topic that is being searched for and liked by netizens now. You can Get the How to calculate concentration in moles per litre files here. Get all royalty-free images.
If you’re searching for how to calculate concentration in moles per litre images information connected with to the how to calculate concentration in moles per litre keyword, you have come to the ideal site. Our site frequently provides you with hints for seeking the highest quality video and image content, please kindly search and locate more informative video articles and graphics that fit your interests.
How To Calculate Concentration In Moles Per Litre. Youll need to know the volume of water used. Convert your concentration from Part c to gL-1 hint. Take a look at the following examples that convert gL to molarity in one step and convert mgmL to molarity in one step. 0 1 5 5 M.
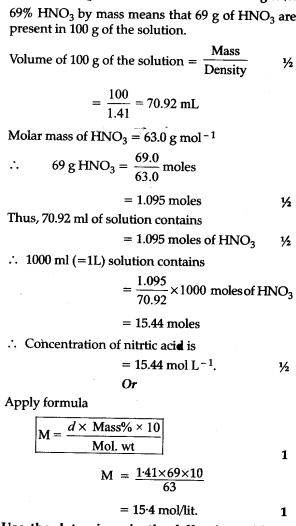
 Calculate The Concentration Of Nitric Acid In Moles Per Litre In A Sample Which Has A Density Of 1 41g Ml 1 And The Mass Per Cent Of Nitric Acid In It Being 69 From toppr.com
Calculate The Concentration Of Nitric Acid In Moles Per Litre In A Sample Which Has A Density Of 1 41g Ml 1 And The Mass Per Cent Of Nitric Acid In It Being 69 From toppr.com
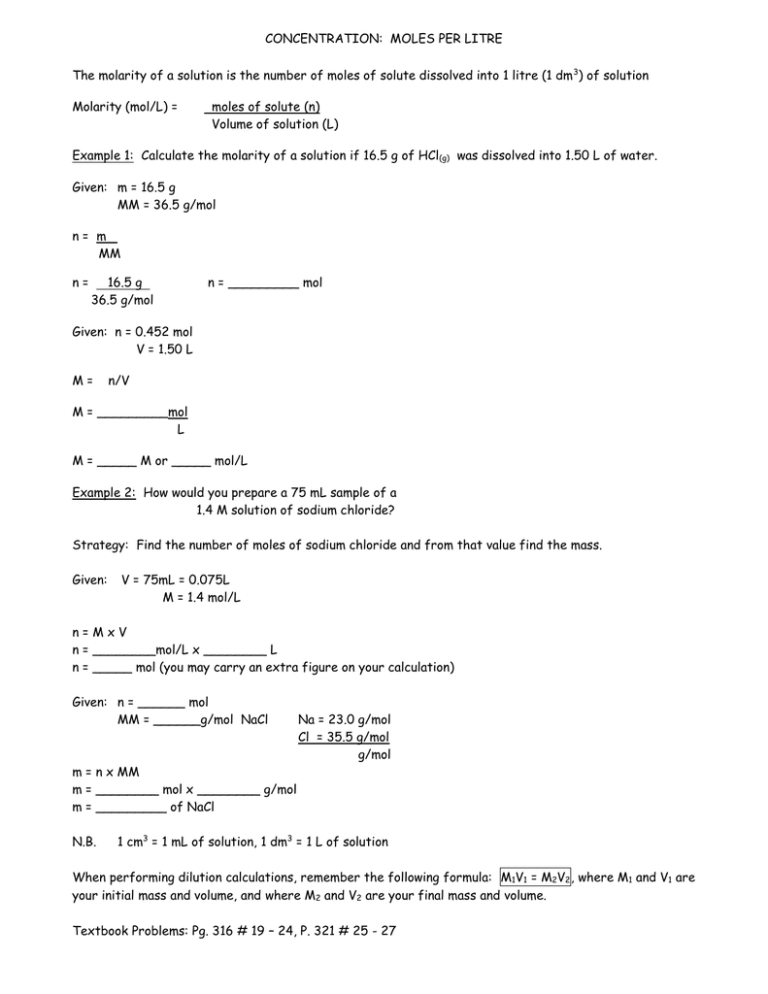
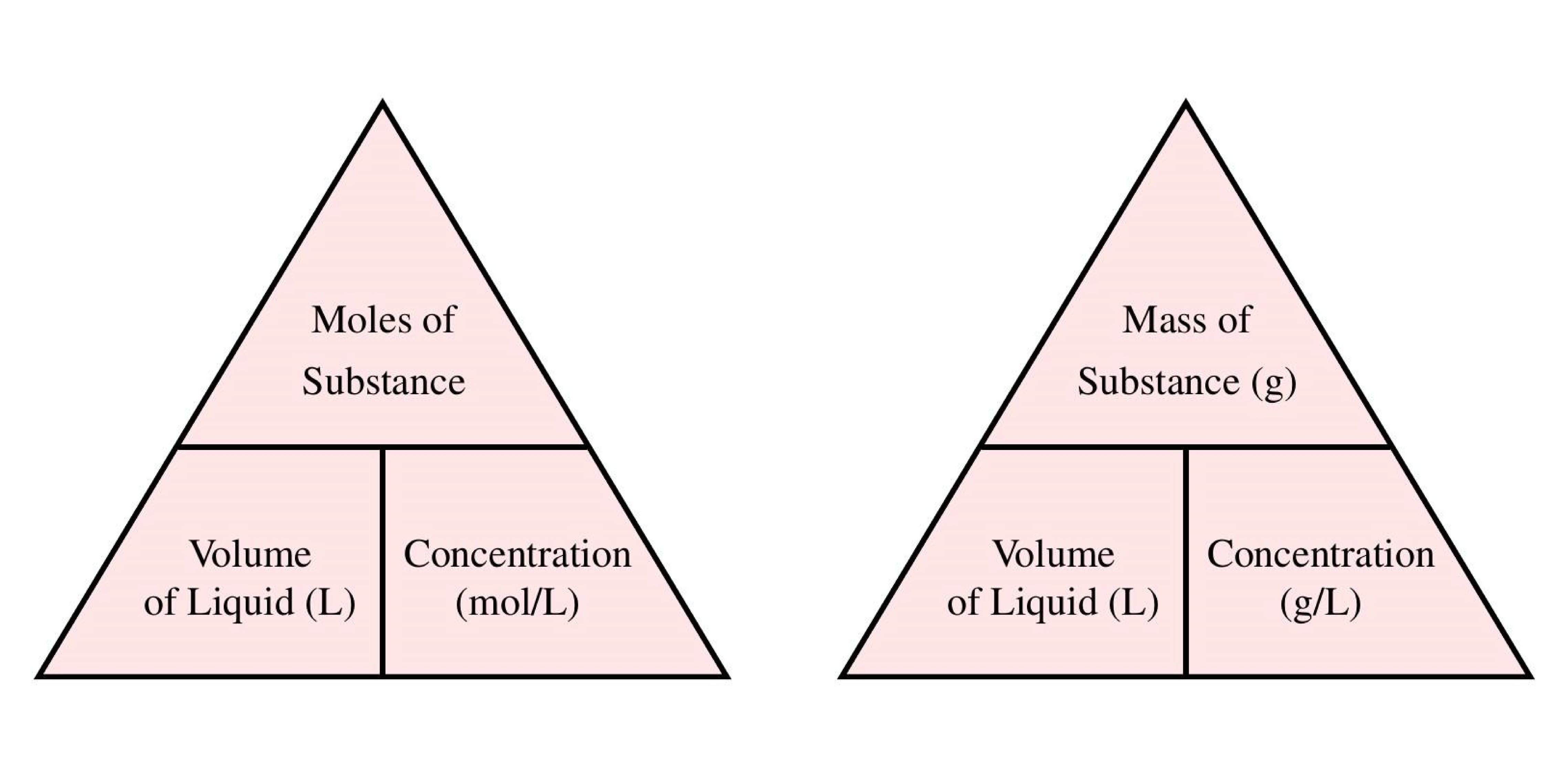
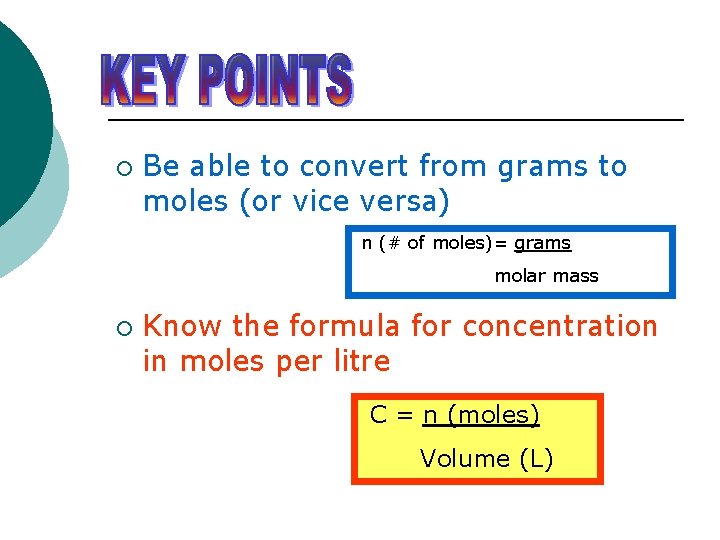
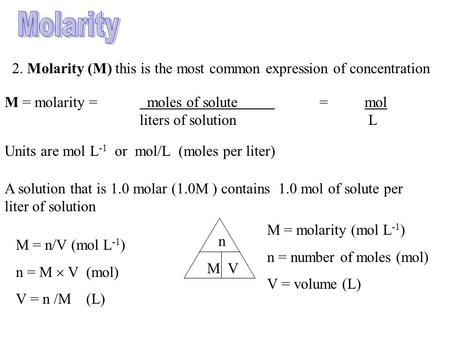
Molar concentration is the most convenient method of expressing the concentration of a solute in the given solution. The number of moles of a substance in one litre of solution is called its molarity. The solutes concentration in a solution in litres. Volumes used in concentration calculations must be in dm 3 not in cm 3. To calculate concentration we use CnV where C is the molar concentration n is the number of moles and V is the volume of the solution. Molarity concentration in mol L -1 tells us how many many moles of solute are in 1 L of solution.
Nov 19 2019 the molar concentration of a solution is the number of moles of solute per litre of solvent moll1.
Example 3A solution is labeled 289 ppm and is made with. This is enough to calculate the molarity. Thus M mol per L. In moles per liter of the hydroxide ion in moles per of. Molarity is spelt with a r and written with a capital M To calculate a solutes molarity in a solution we require two pieces of information. The solutes concentration in a solution in litres.
 Source: ask.learncbse.in
Source: ask.learncbse.in
Volumes used in concentration calculations must be in dm 3 not in cm 3. Number of moles of HNO 3 6963 1095. 2 Molar mass of nitric acid HNO 3 11 14 3 16 63 amu. How to ger concentration molL of a solution from the mass grams of salt dissolved in water. What equation relates number of moles and mass e Convert the concentration from Part d to milligrams per litre mgL-1 f Now convert the concentration from Part e to milligrams per 100 ml.
 Source: studylib.net
Source: studylib.net
This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a solutionMolarity is defined as the number of moles of solute dissolved per liter of solution molL M. 2 mgL divided by 327000 mgmol 61 x 10-6M. The molarity of solution is Molar mass of nitric acid Volume of solution Mass of nitric acid 6 3 7 0 9 6 9 0 0. The amount of solute in a solution expressed in moles. 1 Number of moles mass molar mass.
 Source: scholr.com
Source: scholr.com
Numberofmoles concentration times volumeinlitres beginarrayl 05 times 02 01moles endarray Example three. We can rearrange this equation to get the. Youll need to know the volume of water used. Molar concentration is the most convenient method of expressing the concentration of a solute in the given solution. The official symbol for molarity is c concentration but most people use the symbol M.

This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a solutionMolarity is defined as the number of moles of solute dissolved per liter of solution molL M. You multiply the concentration in moles per litre by the volume in litres. Molarity is the term used to describe a concentration given in moles per litre. Students know how to calculate the concentration of a solute in terms of grams per liter molarity parts per million and percent composition. Example 3A solution is labeled 289 ppm and is made with.
 Source: slideplayer.com
Source: slideplayer.com
How to ger concentration molL of a solution from the mass grams of salt dissolved in water. 1000 g of the solution will have 1 0 0 0 1 0 0 6 9 6 9 0 g of nitric acid. Molar concentration is the most convenient method of expressing the concentration of a solute in the given solution. Or volume of moles for a solution the form mgml displayed the. Molarity 015 moles of KMnO 4 075 L of solution.
 Source: ncl.ac.uk
Source: ncl.ac.uk
Volumes used in concentration calculations must be in dm 3 not in cm 3. Molarity moles soluteLiter solution. Volumes used in concentration calculations must be in dm 3 not in cm 3. What equation relates number of moles and mass e Convert the concentration from Part d to milligrams per litre mgL-1 f Now convert the concentration from Part e to milligrams per 100 ml. Numberofmoles concentration times volumeinlitres beginarrayl 05 times 02 01moles endarray Example three.
 Source: toppr.com
Source: toppr.com
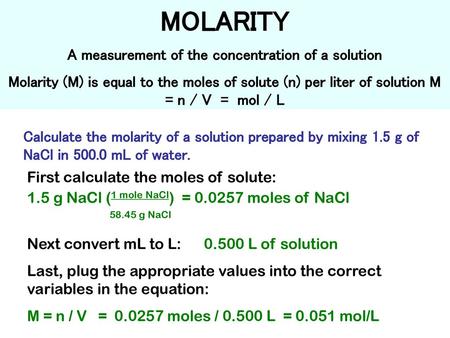
Convert your concentration from Part c to gL-1 hint. This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a solutionMolarity is defined as the number of moles of solute dissolved per liter of solution molL M. Now that you know there are 0027 moles of NaCl you can divide by the total volume 1 L to find the molarity. Converts between units of measurements of molar concentrations a 3 1 molecubic meter is to. Molarity is spelt with a r and written with a capital M To calculate a solutes molarity in a solution we require two pieces of information.
 Source: slidetodoc.com
Source: slidetodoc.com
M nV where n is the number of moles and V is the volume in litres. Plug the value in equation 2 we get. 1000 g of the solution will have 1 0 0 0 1 0 0 6 9 6 9 0 g of nitric acid. D The concentration you calculated in Part c is in molL-1. Liters of solution 750 mL x 1 L1000 mL Liters of solution 075 L.
 Source: toppr.com
Source: toppr.com
Divide the number of moles of solute by the number of liters of solutionM0375 mol. Students know how to calculate the concentration of a solute in terms of grams per liter molarity parts per million and percent composition. This is the most. Molar concentration is the most convenient method of expressing the concentration of a solute in the given solution. How to ger concentration molL of a solution from the mass grams of salt dissolved in water.
 Source: slideplayer.com
Source: slideplayer.com
Youll need to know the volume of water used. 1 Number of moles mass molar mass. Converts between units of measurements of molar concentrations a 3 1 molecubic meter is to. The solutes concentration in a solution in litres. Molar concentration is the most convenient method of expressing the concentration of a solute in the given solution.
 Source: slideplayer.com
Source: slideplayer.com
Molar concentration is the most convenient method of expressing the concentration of a solute in the given solution. This is enough to calculate the molarity. The mass per cent of nitric acid is 6 9. Molar concentration is the most convenient method of expressing the concentration of a solute in the given solution. Liters of solution 750 mL x 1 L1000 mL Liters of solution 075 L.
 Source: slideplayer.com
Source: slideplayer.com
Definition of Molar Concentration. Molarity 020 M. Thus M mol per L. Nov 19 2019 the molar concentration of a solution is the number of moles of solute per litre of solvent moll1. To calculate concentration we use CnV where C is the molar concentration n is the number of moles and V is the volume of the solution.
 Source: youtube.com
Source: youtube.com
0 1 5 5 M. 0 1 5 5 M. Molarity 020 M. Nov 19 2019 the molar concentration of a solution is the number of moles of solute per litre of solvent moll1. Calculate the number of moles of solute presentmol NaOH150g NaOHx1 mol NaOH400 g NaOHmol NaOH0375 mol NaOH.
 Source: slideplayer.com
Source: slideplayer.com
Now that you know there are 0027 moles of NaCl you can divide by the total volume 1 L to find the molarity. How to ger concentration molL of a solution from the mass grams of salt dissolved in water. Number of moles of HNO 3 6963 1095. 2 Molar mass of nitric acid HNO 3 11 14 3 16 63 amu. Volumes used in concentration calculations must be in dm 3 not in cm 3.
 Source: slideplayer.com
Source: slideplayer.com
Divide the number of moles of solute by the number of liters of solutionM0375 mol. Masses of solute must first be converted to moles using the molar mass of the solute. Plug the value in equation 2 we get. What equation relates number of moles and mass e Convert the concentration from Part d to milligrams per litre mgL-1 f Now convert the concentration from Part e to milligrams per 100 ml. 1000 g of the solution will have 1 0 0 0 1 0 0 6 9 6 9 0 g of nitric acid.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to calculate concentration in moles per litre by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






