Your How to calculate 1 mole of nacl images are available. How to calculate 1 mole of nacl are a topic that is being searched for and liked by netizens now. You can Download the How to calculate 1 mole of nacl files here. Download all royalty-free images.
If you’re searching for how to calculate 1 mole of nacl images information related to the how to calculate 1 mole of nacl interest, you have visit the right site. Our website always provides you with hints for seeing the highest quality video and image content, please kindly search and find more informative video articles and images that match your interests.
How To Calculate 1 Mole Of Nacl. Mole number of NaCl Sodium Chloride grams weight of NaCl Molecular mass of NaCl. Now we have to divide the gram weight by molecular mass of Sodium Chloride. 1 grams NaCl is equal to 0017110756386119 mole. Moles 50585 0855 moles 3sf.
 Mole Calculations Moles To Mass Find The Mass Of One Mole Of Nacl 58 5g Find The Mass Of Two Moles Of Nacl 117 0g How Did You Get The Answer 2 Moles From slideplayer.com
Mole Calculations Moles To Mass Find The Mass Of One Mole Of Nacl 58 5g Find The Mass Of Two Moles Of Nacl 117 0g How Did You Get The Answer 2 Moles From slideplayer.com
M 062 moles NaCl 050 liter solution 12 M solution 12 molar solution. 60231023 of Cl. NaCl 229898 gL 354530 gL. Mole number of NaCl Sodium Chloride grams weight of NaCl Molecular mass of NaCl. Molecular weight of NaCl or mol. Consequently how do you calculate Osmole.
125 g NaCl.
If you dont believe me look at the equation Na Cl - NaCl and look at the coefficients. How do you find the equivalent weight of NaCl. M 062 moles NaCl 050 liter solution 12 M solution 12 molar solution. Mole number of NaCl Sodium Chloride grams weight of NaCl Molecular mass of NaCl. You can view more details on each measurement unit. Moles 50585 0855 moles 3sf.
 Source: slideplayer.com
Source: slideplayer.com
A mole is 60231023. A 1 molar solution is a solution in which 1 mole of a compound is dissolved in a total volume of 1 litre. Quick conversion chart of moles NaCl to grams. Mass of NaCl is 75g Formula. Molar mass of NaCl is 584428 gmol.
 Source: youtube.com
Source: youtube.com
Divide the molecular weight by the valence to calculate the equivalent weight. For example the molar mass of NaCl can be calculated for finding the atomic mass of sodium 2299 gmol and the atomic mass of chlorine 3545 gmol and combining them. 1 grams NaCl is equal to 0017110756386119 mole. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. 1 moles NaCl to grams 5844277 grams.
 Source: topperlearning.com
Source: topperlearning.com
1 mole of NaCl 23355585g. The molar mass of NaCl is 5844g and thus the number of moles can be easily calculated. The SI base unit for amount of substance is the mole. So in this way the mass of one mole of NaCl is the mass of Na and mass of Cl. This compound is also known as Sodium Chloride.
 Source: youtube.com
Source: youtube.com
You can view more details on each measurement unit. NaCl 229898 gL 354530 gL. 60231023 atoms of Na 1 mol of Na. Therefore NaCl 584538 gL. 60231023 of Cl.
 Source: clutchprep.com
Source: clutchprep.com
Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom. 3 moles NaCl to grams 17532831 grams. The SI base unit for amount of substance is the mole. If you dont believe me look at the equation Na Cl - NaCl and look at the coefficients. How to Calculate the Number of Moles in a Solution.
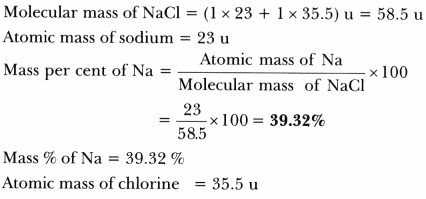 Source: ask.learncbse.in
Source: ask.learncbse.in
Relative formula mass M r is found by adding together the relative atomic masses A r of all the atoms present in a compound. 1 mole of NaCl 23355585g. Moles 50585 0855 moles 3sf. If you dissolve 5844g of NaCl in a final volume of 1 litre you have made a 1M NaCl solution. 1 moles NaCl to grams 5844277 grams.
 Source: brainly.in
Source: brainly.in
2 moles NaCl to grams 11688554 grams. Relative formula mass M r is found by adding together the relative atomic masses A r of all the atoms present in a compound. NaCl 229898 gL 354530 gL. Molar mass of NaCl 22989Na 35453Cl 58442 gmol Number of mole 75g 5844gmol 128 mol. Quick conversion chart of moles NaCl to grams.
 Source: slideplayer.com
Source: slideplayer.com
Therefore if you have a mole of NaCl multiplying the ratio by the number will give you 60231023 Na. 1 mOsm of Na and 1 mOsm of Cl. Moles 50585 0855 moles 3sf. Put the number of grams in the molar mass on the bottom. Mole number of NaCl Sodium Chloride grams weight of NaCl Molecular mass of NaCl.
 Source: chemiris.labs.brocku.ca
Source: chemiris.labs.brocku.ca
Therefore if you have a mole of NaCl multiplying the ratio by the number will give you 60231023 Na. Suppose the molecular weight of NaCl is M NaCl. This compound is also known as Sodium Chloride. Now we have to divide the gram weight by molecular mass of Sodium Chloride. Most noteworthy each molecule has 1 Na Sodium and 1 Cl Chloride atom.
 Source: slideplayer.com
Source: slideplayer.com
This means you have around 123 mol of NaCl in 7233 grams of it. Put the number of grams in the molar mass on the bottom. We assume you are converting between grams NaCl and mole. 1 mole of a substance contains 60231023 noof molecules. Now we have to divide the gram weight by molecular mass of Sodium Chloride.
 Source: youtube.com
Source: youtube.com
How do you find the equivalent weight of NaCl. Put 1 mol on the top. The answer is 5844277. 18g NaClmolar mass NaCl moles NaCl. 1 mOsm of Na and 1 mOsm of Cl.
 Source: youtube.com
Source: youtube.com
Suppose the molecular weight of NaCl is M NaCl. Molar mass of NaCl is 584428 gmol. Volume 100 gdensity and convert mL to L. Putting all of this into our moles equation gives us the answer. Divide the molecular weight by the valence to calculate the equivalent weight.
 Source: toppr.com
Source: toppr.com
The SI base unit for amount of substance is the mole. 60231023 of Cl. 2 moles NaCl to grams 11688554 grams. You can view more details on each measurement unit. Type in your own numbers in the form to convert the units.
 Source: slideplayer.com
Source: slideplayer.com
Click to see full answer. How to Calculate the Number of Moles in a Solution. Moles 50585 0855 moles 3sf. NaCl Na Cl. For example assuming that NaCl completely dissociates into Na and Cl in solution each millimole of NaCl provides 2 milliosmoles mOsm.
 Source: people.tamu.edu
Source: people.tamu.edu
The molecular weight of sodium chloride NaCl is 5844 so one gram molecular weight 1 mole is 5844g. Now we have to divide the gram weight by molecular mass of Sodium Chloride. 2 moles NaCl to grams 11688554 grams. Use this page to learn how to convert between moles NaCl and gram. But each Na ion pairs with a negative ion X such as Cl to give 2 Osmol of particles.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to calculate 1 mole of nacl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






