Your How much mass does one mole of o2 gas have images are ready in this website. How much mass does one mole of o2 gas have are a topic that is being searched for and liked by netizens now. You can Get the How much mass does one mole of o2 gas have files here. Get all free vectors.
If you’re looking for how much mass does one mole of o2 gas have images information connected with to the how much mass does one mole of o2 gas have keyword, you have visit the ideal blog. Our site frequently provides you with suggestions for seeking the maximum quality video and image content, please kindly hunt and locate more informative video articles and images that match your interests.
How Much Mass Does One Mole Of O2 Gas Have. 373 gmol 373 gmol 835 gmol 224 gmol. We know that 1 mol of oxygen gas contains 2 moles of O. And one mole of oxygen molecules has a mass of 32g so three moles would have a mass of 96g. But ceO2 is greater than ceH2 in size I asked my teacher why the volume occupied by 1 mole of ceH_2 is equal to the volume occupied by ceO_2 at standard temperature and pressure but he seemed to not know the answer and I remain confused.
 Selinaconcise Chemistryclass10 Icsesolutions Moleconceptandstoichiometry Aplustopper Chemistry Class Mole Concept Chemistry From in.pinterest.com
Selinaconcise Chemistryclass10 Icsesolutions Moleconceptandstoichiometry Aplustopper Chemistry Class Mole Concept Chemistry From in.pinterest.com
For those elements that do not occur as single atoms - that is the diatomic gases sulfur and phosphorus - it is important to be certain that you specify what you are talking about. However you would never get moles of oxygen atoms as oxygen always pairs up into molecules of O2. Oxygen gas is diatomic meaning two atoms of 16 gmol each so 1 mole of O2 has a mass of 32 grams. Based on that estimate for the weight of atmosphere the total quantity of O2 in atmosphere is around 5102132 15625e20 mole. 0827 00821 361 128 0270 2451 03456. Oyxgen is a dyatomic molecule thats why we have 2 moles of O.
In the case of moles however this definition doesnt help much.
That means that there are two atoms of oxygen. Since both of these elements are diatomic in air - O2 and N2 the molar mass of oxygen gas is aprox. 1 moles O2 319988 gram using the molecular weight calculator and the molar mass of O2. Divide the amount you have by the molar weight to obtain the number of moles in the sample. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 1023atoms of oxygen. 373 gmol 373 gmol 835 gmol 224 gmol.
 Source: slideplayer.com
Source: slideplayer.com
Since both of these elements are diatomic in air - O2 and N2 the molar mass of oxygen gas is aprox. 373 gmol 373 gmol 835 gmol 224 gmol. One mole of oxygen gas has a mass of _____ g. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. The atomic mass of carbon is 12 grams per mole.
 Source: youtube.com
Source: youtube.com
However as you note that mole of O X 2 gas actually expands to fill a full 224 L of space at STP. And one mole of oxygen molecules has a mass of 32g so three moles would have a mass of 96g. 373 gmol 373 gmol 835 gmol 224 gmol. One mole of oxygen gas has a mass of _____ g. Another example can be.
 Source: pinterest.com
Source: pinterest.com
One mole of any gas has a volume of 24 dm 3 or 24000 cm 3 at rtp room temperature and pressure. A mole is a unit of measurement that is. The atomic mass of carbon is 12 grams per mole. So one mole of O 2 weights. However as you note that mole of O X 2 gas actually expands to fill a full 224 L of space at STP.
 Source: youtube.com
Source: youtube.com
32 gmol and the molar mass of nitrogen gas is aprox. How much mass does 1 mole of O2 gas have. That means that there are two atoms of oxygen. 32 gmol and the molar mass of nitrogen gas is aprox. One mole of oxygen atoms has a mass of 16g.
 Source: pinterest.com
Source: pinterest.com
So that space is actually only about 32 c m 3 224 L 014 full of O X 2. And one mole of oxygen molecules has a mass of 32g so three moles would have a mass of 96g. So three moles of oxygen atoms would have a mass of 48g. One mole of oxygen gas has a mass of _____ g. For one mole of O X 2 molecules that works out to just under 32 c m 3 m o l of volume actually occupied by the oxygen molecules themselves.
 Source: youtube.com
Source: youtube.com
Based on that estimate for the weight of atmosphere the total quantity of O2 in atmosphere is around 5102132 15625e20 mole. So that space is actually only about 32 c m 3 224 L 014 full of O X 2. In the case of moles however this definition doesnt help much. 24 grams 32 gramsmol 075 moles. Based on that estimate for the weight of atmosphere the total quantity of O2 in atmosphere is around 5102132 15625e20 mole.
 Source: youtube.com
Source: youtube.com
1 mol of oxygen weighs 16 gmol the mass for 1 molecule of O. A mole is a unit of measurement that is. Mass of one molecule of oxygen 32 Avogadro constant 602214076 10 23 531 10-23g. Another example can be. Do a quick conversion.
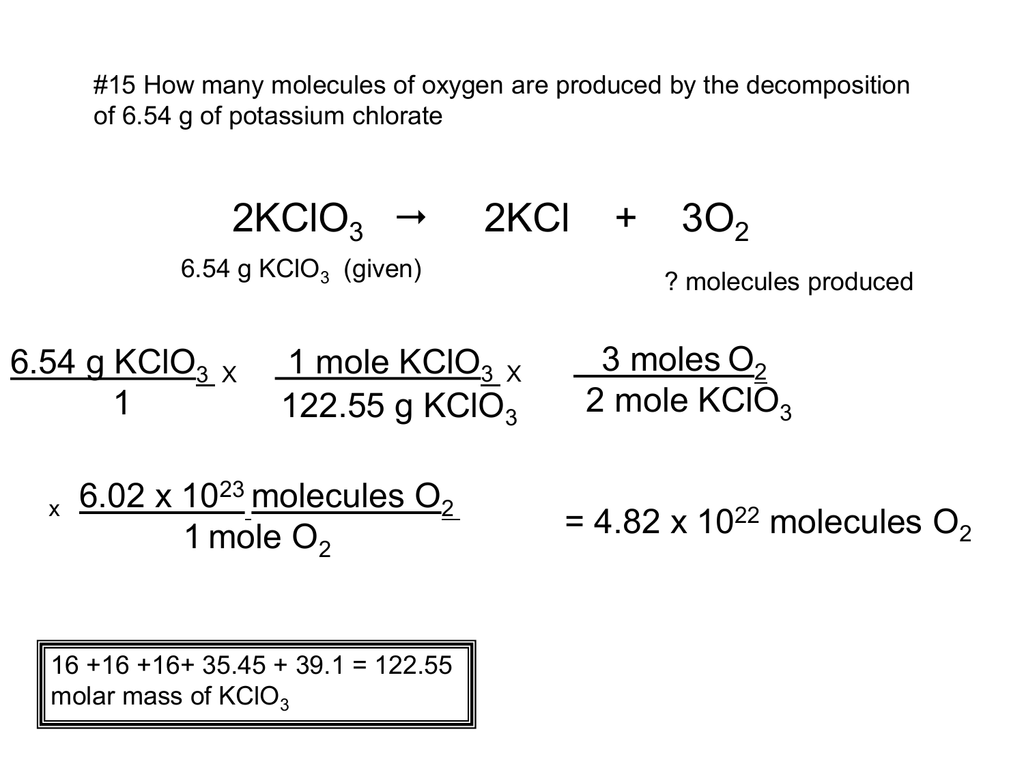 Source: studylib.net
Source: studylib.net
Oxygen gas is diatomic meaning two atoms of 16 gmol each so 1 mole of O2 has a mass of 32 grams. So one mole of O 2 weights. The atomic mass of carbon is 12 grams per mole. What is the molar mass molecular weight of the gas. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie.
 Source: in.pinterest.com
Source: in.pinterest.com
Density of oxygen is equal to 1429 kgm³. Avogadros number N_A 6022140857xx1023. A 0856 L sample of a gas has a mass of 319 g at STP. One mole of oxygen gas has a mass of _____ g. For those elements that do not occur as single atoms - that is the diatomic gases sulfur and phosphorus - it is important to be certain that you specify what you are talking about.
 Source: youtube.com
Source: youtube.com
That means that there are two atoms of oxygen. That means that there are two atoms of oxygen. Do a quick conversion. One mole of oxygen gas which has the formula O 2 has a mass of 32 g and contains 602 X 10 23 molecules of oxygen but 1204 X 10 23 2 X 602 X 10 23 atoms because each molecule of oxygen contains two oxygen atoms. So one mole of O 2 weights.
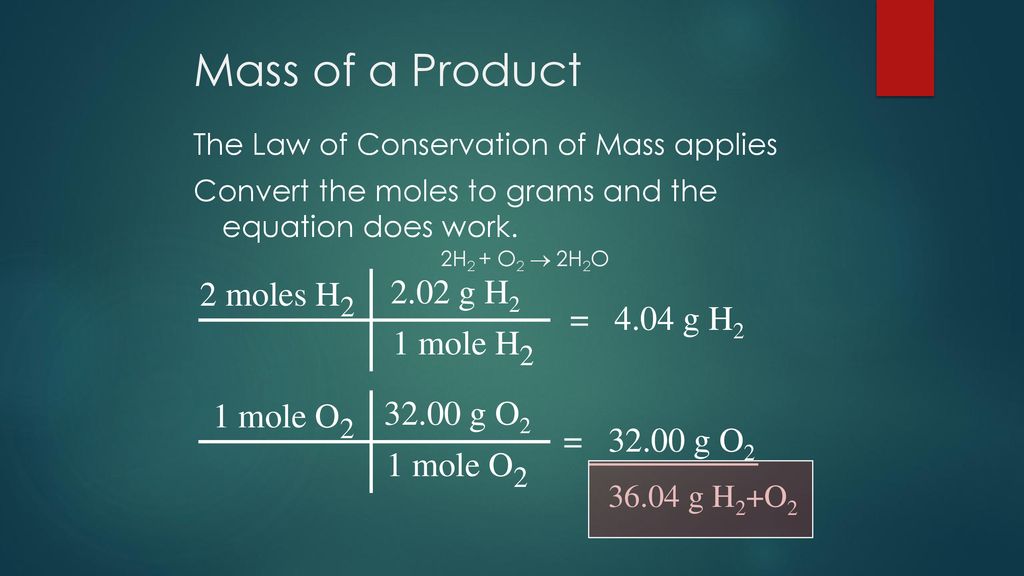 Source: slideplayer.com
Source: slideplayer.com
However as you note that mole of O X 2 gas actually expands to fill a full 224 L of space at STP. A 0856 L sample of a gas has a mass of 319 g at STP. Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol. For one mole of O X 2 molecules that works out to just under 32 c m 3 m o l of volume actually occupied by the oxygen molecules themselves. Oxygen gas is diatomic meaning two atoms of 16 gmol each so 1 mole of O2 has a mass of 32 grams.
 Source: youtube.com
Source: youtube.com
One mole of oxygen gas which has the formula O 2 has a mass of 32 g and contains 602 X 10 23 molecules of oxygen but 1204 X 10 23 2 X 602 X 10 23 atoms because each molecule of oxygen contains two oxygen atoms. This volume is called the molar volume of a. So that space is actually only about 32 c m 3 224 L 014 full of O X 2. In 1 mol O_2 gas by definition you have Avogadros number of MOLECULES. What is the molar mass molecular weight of the gas.
 Source: youtube.com
Source: youtube.com
1 mol of oxygen weighs 16 gmol the mass for 1 molecule of O. A mole is a unit of measurement that is. One mole of any gas has a volume of 24 dm 3 or 24000 cm 3 at rtp room temperature and pressure. Avogadros number N_A 6022140857xx1023. However as you note that mole of O X 2 gas actually expands to fill a full 224 L of space at STP.
 Source: pinterest.com
Source: pinterest.com
One mole of oxygen gas has a mass of _____ g. One mole of oxygen gas has a mass of _____ g. By the way the mass for 1 mol of O₂ may be. This volume is called the molar volume of a. In 1 mol O_2 gas by definition you have Avogadros number of MOLECULES.

24 grams 32 gramsmol 075 moles. However as you note that mole of O X 2 gas actually expands to fill a full 224 L of space at STP. For one mole of O X 2 molecules that works out to just under 32 c m 3 m o l of volume actually occupied by the oxygen molecules themselves. Since both of these elements are diatomic in air - O2 and N2 the molar mass of oxygen gas is aprox. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how much mass does one mole of o2 gas have by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






