Your How much is 1 mole of water molecules answers images are available in this site. How much is 1 mole of water molecules answers are a topic that is being searched for and liked by netizens now. You can Download the How much is 1 mole of water molecules answers files here. Download all royalty-free photos.
If you’re searching for how much is 1 mole of water molecules answers pictures information related to the how much is 1 mole of water molecules answers interest, you have visit the right site. Our site always gives you hints for seeing the highest quality video and picture content, please kindly surf and locate more informative video content and images that match your interests.
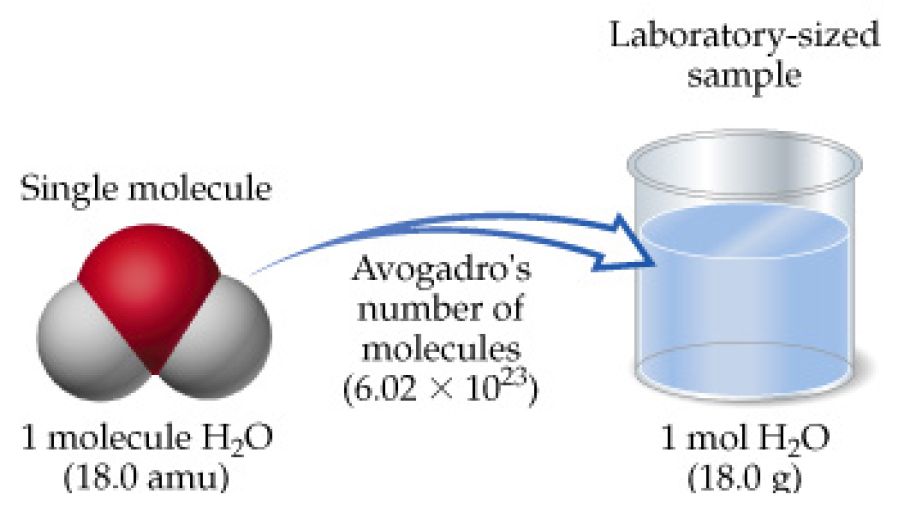
How Much Is 1 Mole Of Water Molecules Answers. This answer will have 3 SF 540 g601 g00185 g0167 g. This means that one mole of water molecules has a mass of 18015 g. 27144moles H2O 60221023molec1mole H2O 16351024molec. But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x 10 24 atoms in a mole of water.
 How Many Molecules Are In 48 90 Grams Of Water Socratic From socratic.org
How Many Molecules Are In 48 90 Grams Of Water Socratic From socratic.org
Molecules in a drop of water 6022 x 10 23 moleculesmole x 0002275 moles. Therefore if you have 52 moles of water naturally you will have 52 moles x 6023 x 1023 water molecules mole 31 x1024 water molecules. 120 g of hydrogen gas correct 25 g of an unknown compound 3100 g of water 41020 hydrogen atoms 5100g of a substance thatis 2 H by mass 1020 H atoms is much less than 1. So in a cubic meter of air there is 2927 grams of water vapor per cubic meter at sea level. Click to see full answer. But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x 10 24 atoms in a mole of water.
Hence the volume should be.
One mol of NaCl 602 x1023 formulas has a mass of 5844 g. This of course means that two molecules of water will have a smaller mass than two molecules of sugar. This answer will have 3 SF 540 g601 g00185 g0167 g. The same goes for 10 molecules of each substance 100 1000 and 6022 1023. Mol is the base unit of amount of substance in the International System of Units SI. The mass of 0100 mole of neon is 2018 grams.
 Source: socratic.org
Source: socratic.org
Therefore there will be 60221023 oxygen atoms in 1 mole or 18 g of water. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs. There are therefore 602 10 23 water molecules in a mole of water molecules. Subsequently question is how much oxygen is in 100 grams of water. Weight of water 20158 g 159994 g.
 Source: toppr.com
Source: toppr.com
Please remember the Avogadros Number which states that 1 mole of a substance water in your case contains 6023 x 1023 molecules. It is defined as exactly 6022 140 76 10 23 elementary entities particles which may be atoms molecules ions or electrons. True False Question 2MultipleChoice Score. 120 g of hydrogen gas correct 25 g of an unknown compound 3100 g of water 41020 hydrogen atoms 5100g of a substance thatis 2 H by mass 1020 H atoms is much less than 1. The relation between molecular formula mass and molar mass.
 Source: pinterest.com
Source: pinterest.com
The relation between molecular formula mass and molar mass. Weight of water 180152 g. It is defined as exactly 6022 140 76 10 23 elementary entities particles which may be atoms molecules ions or electrons. As the molar mass of hydrogen is 2 it requires 20018 1009 g of hydrogen. Over 224 liters that is 080424 kgm3.
 Source: toppr.com
Source: toppr.com
The mass of 0100 mole of neon is 2018 grams. 100 mg x 1 mole180 grams x 1 gram1000 mg x 1 liter05 moles x 1000 mlliter 111 ml If you were to dilute 100 ml of the 05M glucose solution with 400 ml water. But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x 1024 atoms in a mole of water. 05 moleliter x 1 liter x 6023x1023 moleculesmole 3012x1023 molecules same number of molecules How much of the 05M glucose solution is needed to provide 100 mg of glucose. As the molar mass of hydrogen is 2 it requires 20018 1009 g of hydrogen.
 Source: pinterest.com
Source: pinterest.com
Hence the volume should be. This means that one mole of water molecules has a mass of 18015 g. Thus for example one mole of water contains 60221407610 23 molecules whose total mass is about 18015 grams and the mean mass of one molecule of water is about 18015 daltons. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. One mol of NaCl 602 x1023 formulas has a mass of 5844 g.
 Source: in.pinterest.com
Source: in.pinterest.com
Hence the volume should be. Water has a molar mass of 18015 gmol. This answer will have 3 SF 540 g601 g00185 g0167 g. True False Question 2MultipleChoice Score. 120 g of hydrogen gas correct 25 g of an unknown compound 3100 g of water 41020 hydrogen atoms 5100g of a substance thatis 2 H by mass 1020 H atoms is much less than 1.
 Source: pinterest.com
Source: pinterest.com
A cubic meter of air near saturation may contain 28 grams or about 16 moles of water molecules at 30 C but only 8 grams or about 044 mole of water molecules per cubic meter of air at 8 C. The relation between molecular formula mass and molar mass. The definition of mole was adopted in November 2018 as one of the seven SI base units revising the previous definition that specified one mole as. Atomic mass is the number of grams per mole of the element. The mass of 0100 mole of neon is 2018 grams.
 Source: pinterest.com
Source: pinterest.com
Weight of water 20158 g 159994 g. A mole of water has 18015 grams of water. Weight of water 180152 g. Secondly how many molecules are in a mole. One mole of water contains 602 x 10 23 MOLECULES of water.
 Source: pinterest.com
Source: pinterest.com
The same goes for 10 molecules of each substance 100 1000 and 6022 1023. Weight of water 20158 g 159994 g. Molecules in a drop of water 6022 x 10 23 moleculesmole x 0002275 moles. So to sum this up 60221023 molecules of water will amount to 1 mole of water which in turn will have a mass of 18015 g. The mole is widely used in chemistry as a convenient way to express amounts of reactants and products of chemical reactions.
 Source: youtube.com
Source: youtube.com
Over 224 liters that is 080424 kgm3. How many grams of water were formed. Secondly how many molecules are in a mole. The mass of 0100 mole of neon is 2018 grams. Please remember the Avogadros Number which states that 1 mole of a substance water in your case contains 6023 x 1023 molecules.

Atomic mass is the number of grams per mole of the element. This of course means that two molecules of water will have a smaller mass than two molecules of sugar. The definition of mole was adopted in November 2018 as one of the seven SI base units revising the previous definition that specified one mole as. Chemistry 11112020 0100 lightning1157blaze. When 300 moles of hydrogen molecules and 150 moles of oxygen molecules react they form 300 moles of water according to the reaction below.
 Source: slidetodoc.com
Source: slidetodoc.com
V 1 m o l 18 10 3 k g m o l 1 1 10 3 k g m 3 18 10 5 m 3. A mole mol is the amount of a substance that contains 602 10 23 representative particles of that substance. Atomic mass is the number of grams per mole of the element. Hence the volume should be. Weight of water 180152 g.

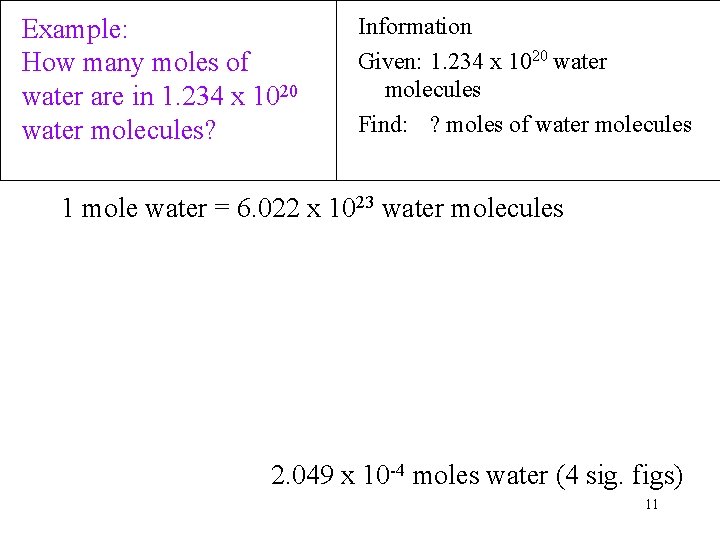
N H 1 mol CH4 602 1023 molec CH4 1 mol CH4 4 H atoms 1 molec CH4 241 1024 H atoms CountingHs 004 100points Which has the greatest number of hydrogen atoms. A mole mol is the amount of a substance that contains 602 10 23 representative particles of that substance. Molecules in a drop of water 167 x 10 21 water molecules. 27144moles H2O 60221023molec1mole H2O 16351024molec. Thus for example one mole of water contains 60221407610 23 molecules whose total mass is about 18015 grams and the mean mass of one molecule of water is about 18015 daltons.
 Source: slidetodoc.com
Source: slidetodoc.com
Therefore if you have 52 moles of water naturally you will have 52 moles x 6023 x 1023 water molecules mole 31 x1024 water molecules. Therefore if you have 52 moles of water naturally you will have 52 moles x 6023 x 1023 water molecules mole 31 x1024 water molecules. Therefore there will be 60221023 oxygen atoms in 1 mole or 18 g of water. Water has a molar mass of 18015 gmol. Hence the volume should be.
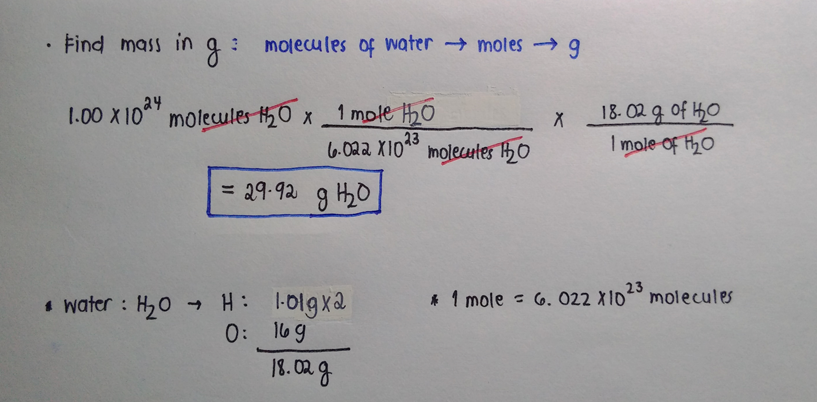 Source: clutchprep.com
Source: clutchprep.com
A mole of water has 18015 grams of water. Therefore if you have 52 moles of water naturally you will have 52 moles x 6023 x 1023 water molecules mole 31 x1024 water molecules. Click to see full answer. 27144moles H2O 60221023molec1mole H2O 16351024molec. The mole is widely used in chemistry as a convenient way to express amounts of reactants and products of chemical reactions.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how much is 1 mole of water molecules answers by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






