Your How much is 1 mole of nacl images are available. How much is 1 mole of nacl are a topic that is being searched for and liked by netizens now. You can Download the How much is 1 mole of nacl files here. Download all royalty-free photos and vectors.
If you’re searching for how much is 1 mole of nacl images information linked to the how much is 1 mole of nacl topic, you have visit the right site. Our site always provides you with hints for seeking the maximum quality video and picture content, please kindly surf and find more enlightening video content and graphics that match your interests.
How Much Is 1 Mole Of Nacl. Molar mass of NaCl 5844277 gmol. One mol of NaCl 602 x1023 formulas has a mass of 5844 g. Since the question asks to make 100 ml not 1 liter we need to make that adjustment. 2298977 35453 Percent composition by element.
 Ions And Molecular Ions Chemistry F Sc Part1 Chapter 1 Lecture 4 Youtube Chemistry Molecular Lecture From pinterest.com
Ions And Molecular Ions Chemistry F Sc Part1 Chapter 1 Lecture 4 Youtube Chemistry Molecular Lecture From pinterest.com
Hence a 1M solution of NaCl contains 5844 g. We assume you are converting between moles NaCl and gram. Calculate the osmolarity of 0140 molL NaCl. That is N 72335844. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams. The molecular weight of sodium chloride NaCl is 5844 so one gram molecular weight 1 mole is 5844g.
The SI base unit for amount of substance is the mole.
So we can say that the number of moles associated with 117g sodium chloride is 002moles. That is N 72335844. Thus the molar mass of NaCl. 1 mole of NaCl is 5844 g 200 g NaCl 1 mol NaCl5844 g NaCl 3422 mol NaCl There are about 34 moles in 200 grams of NaCl. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams. CAS Registry Quantity CAS RN.
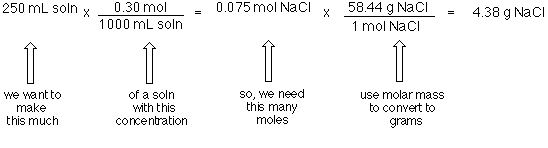 Source: westfield.ma.edu
Source: westfield.ma.edu
People also asked How many moles are in 10 kilograms of NaCl. The molecular weight of NaCl is 585 which means 1 mole of NaCl is 585g. Track your food intake exercise sleep and meditation for free. As an example to make 100 ml of 10 NaCl table salt solution use the previous formula to find out how much NaCl you need. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams.
 Source: slideplayer.com
Source: slideplayer.com
As an example to make 100 ml of 10 NaCl table salt solution use the previous formula to find out how much NaCl you need. 1 mole is equal to 1 moles NaCl or 5844277 grams. Weights of atoms and isotopes are from NIST article. 1 mole is equal to 1 moles NaCl or 5844277 grams. That is N 72335844.
 Source: slideplayer.com
Source: slideplayer.com
H 1 Cl 3545 3645 g. Use this page to learn how to convert between moles NaCl and gram. The molecular weight of NaCl is 585 which means 1 mole of NaCl is 585g. Get control of 2021. 15 moles 15 moles x 5844 gmol 8766 g.
 Source: labpedia.net
Source: labpedia.net
A mole of sodium chloride is 58443 g. Molar mass of NaCl. A mole of sodium chloride is 58443 g. Convert between NaCl weight and moles. HCl is frequently used in enzyme histochemistry.
 Source: in.pinterest.com
Source: in.pinterest.com
Molecular weight of NaCl or grams This compound is also known as Sodium Chloride. Calculate the osmolarity of 0140 molL NaCl. In this video well learn to convert moles of NaCl to grams. One mol of NaCl 602 x1023 formulas has a mass of 5844 g. It will contain 6022 x 1023 chloride ions and 6022 x 1023 sodium ions.
 Source: pinterest.com
Source: pinterest.com
One mol of NaCl 602 x1023 formulas has a mass of 5844 g. In this video well learn to convert moles of NaCl to grams. 1 mole is equal to 1 moles NaCl or 5844277 grams. 1 mole is equal to 1 moles NaCl or 5844277 grams. How many moles of NaCl are present in 1 mole of this solution.
 Source: pinterest.com
Source: pinterest.com
1000 g NaCl x 1 mole NaCl 017110 mol NaCl isolated 58443 g NaCl So 017110 mol NaCl collected x 100 787 yield 02175 mol NaCl theoretical. For our practice problem wel. Sodium chloride commonly known as salt is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. The molar mass of NaCl is 5844g and thus the number of moles can be easily calculated. Thus the molar mass of NaCl.
 Source: pinterest.com
Source: pinterest.com
How many moles of potassium chloride KCl are in 40 L of a 065 M solution. The relation between molecular formula mass and molar mass. 1000 g NaCl x 1 mole NaCl 017110 mol NaCl isolated 58443 g NaCl So 017110 mol NaCl collected x 100 787 yield 02175 mol NaCl theoretical. A solution of 1 molL NaCl has an osmolarity of 2 OsmolL. People also asked How many moles are in 10 kilograms of NaCl.
 Source: pinterest.com
Source: pinterest.com
The technique used can be applied to any mole to gram conversion. NaCl molecular weight. Now you can make your solution. 1 mole of NaCl 5844 grams the molar mass of Na which is 2299 gmol the molar mass of chlorine which is 3545 gmol 5844 gmol. 1 mole is equal to 1 moles NaCl or 5844277 grams.
 Source: slideplayer.com
Source: slideplayer.com
Since the question asks to make 100 ml not 1 liter we need to make that adjustment. 1 mole of NaCl is 5844 g 200 g NaCl 1 mol NaCl5844 g NaCl 3422 mol NaCl There are about 34 moles in 200 grams of NaCl. The GMW of HCl would be the atomic weight of H added to the atomic weight of Cl. Make Your Own Solution for These Projects. H 1 Cl 3545 3645 g.
 Source: pinterest.com
Source: pinterest.com
Get control of 2021. NaCl molecular weight. The SI base unit for amount of substance is the mole. This compound is also known as Sodium Chloride. This means you have around 123 mol of NaCl in 7233 grams of it.
 Source: pinterest.com
Source: pinterest.com
People also asked How many moles are in 10 kilograms of NaCl. The molar mass of NaCl is 5844g and thus the number of moles can be easily calculated. Since the question asks to make 100 ml not 1 liter we need to make that adjustment. Calculate the osmolarity of 0140 molL NaCl. Sodium chloride commonly known as salt is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions.
 Source: pinterest.com
Source: pinterest.com
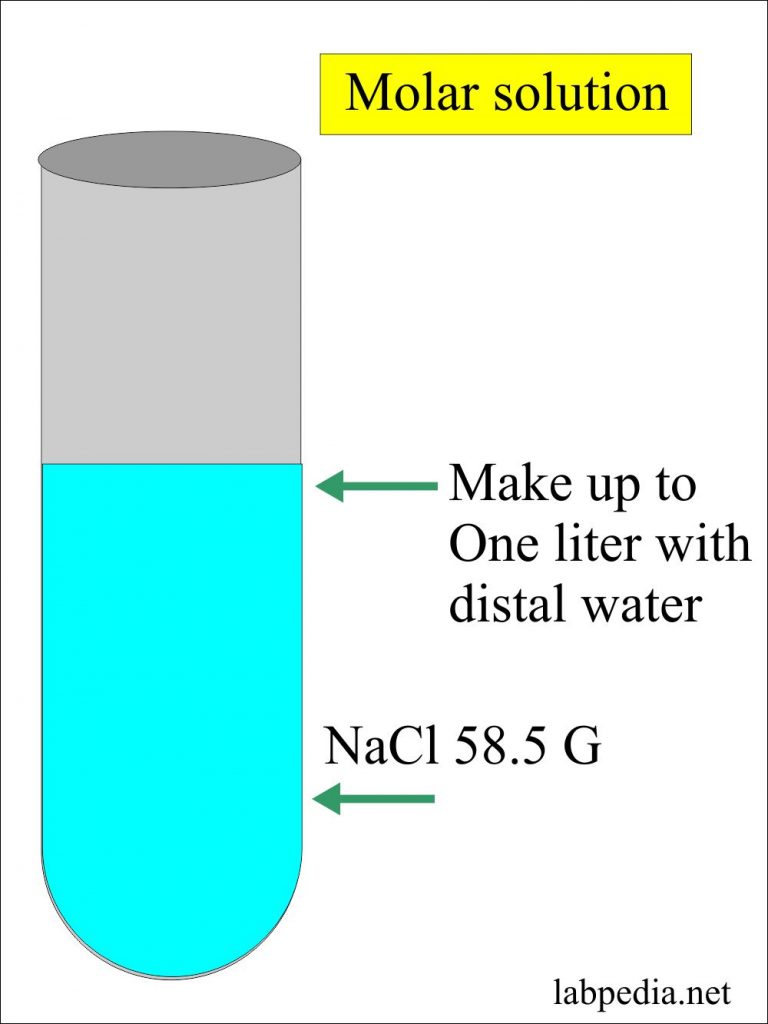
Dissolve 111 g NaCl in 100 ml of water. One mole of Na and one mole of Cl-. Calculate the osmolarity of 0140 molL NaCl. Weights of atoms and isotopes are from NIST article. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
M or Molarity means amount of substance concentration in 1 litre. This means you have around 123 mol of NaCl in 7233 grams of it. 15 moles 15 moles x 5844 gmol 8766 g. How many moles are in a solution. This is the mass needed to make 1 liter 1000 ml.
 Source: slideplayer.com
Source: slideplayer.com
The molecular weight of NaCl is 585 which means 1 mole of NaCl is 585g. Of moles of solute litre of solution Then no. Convert between NaCl weight and moles. 1 mole of NaCl 5844 grams the molar mass of Na which is 2299 gmol the molar mass of chlorine which is 3545 gmol 5844 gmol. Molecular mass molecular weight is the mass of 1 molecule of a substance and is expressed within the unified atomic mass items u.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how much is 1 mole of nacl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






